A kind of detection method of aripiprazole related substance
A technology for the detection of aripiprazole and its detection method, which is applied in the field of drug quality detection, can solve the problems such as the separation degree between the main component and each impurity not meeting the requirements, the damage of the chromatographic column, and the impurity cannot be detected, etc. The effect of good tail factor and small risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
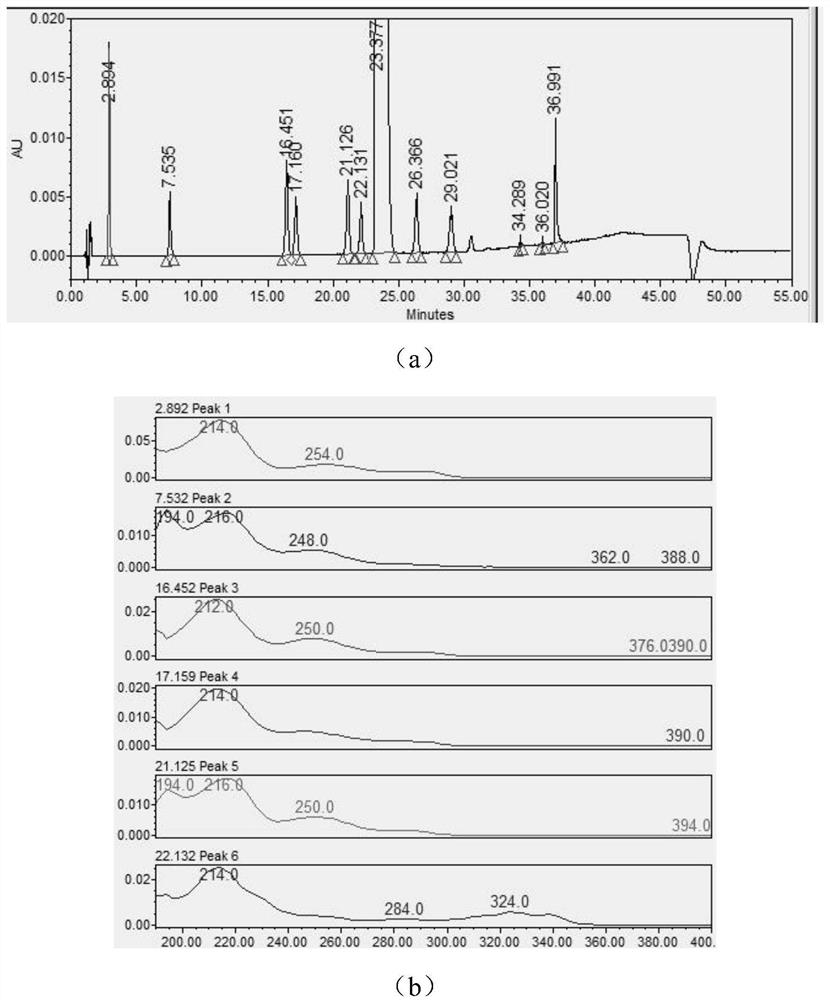
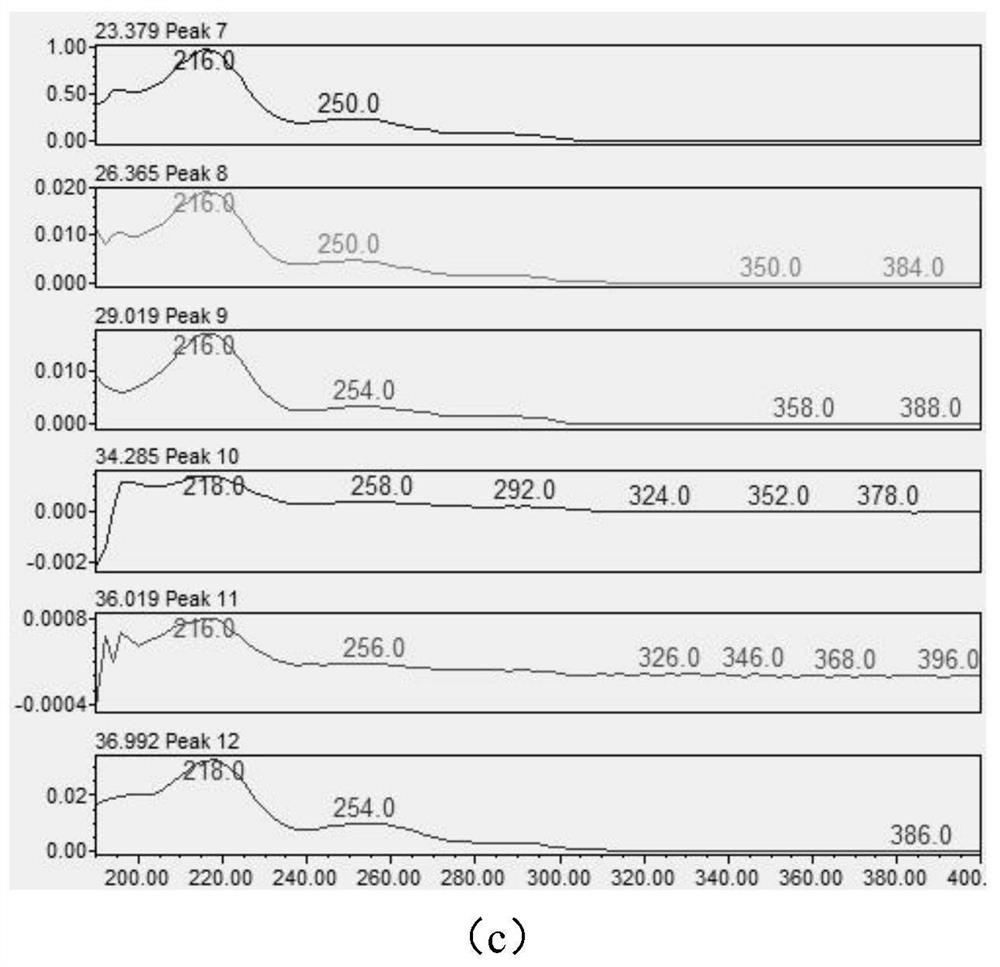
[0084] Utilize ultraviolet-visible spectrophotometer to carry out full-wavelength scanning to aripiprazole and each impurity, obtained result is as follows figure 1 As shown, wherein (a) is the peak-out situation of each impurity and main component in the system suitability solution (preparation method is shown in (2)), and the order of peak-out is EP impurity A, EP impurity B, EP impurity D, EP impurity C, isomer impurity, EP impurity E, aripiprazole, EP impurity F, intermediate I, unknown impurity 1 in the test product, unknown impurity 2 in the test product and EP impurity G, (b) is Full-wavelength scanning results, from top to bottom are EP impurity A, EP impurity B, EP impurity D, EP impurity C, isomer impurities, EP impurity E, (c) is the full-wavelength scanning results, from top to bottom Aripiprazole, EP impurity F, intermediate impurity, unknown impurity 1 in the test product, unknown impurity 2 in the test product and EP impurity G; figure 1 It shows that both arip...
Embodiment 2
[0100] Durability test:
[0101] The preparation method of system suitability solution is identical with embodiment 1;
[0102] The detection wavelength in the reversed-phase high-performance liquid chromatography condition is respectively set to be 249nm and 259nm, and other conditions are consistent with embodiment 1, and the system suitability solution is carried out reversed-phase high-performance liquid chromatography detection, and gained spectrogram is as follows Figure 7 ~ Figure 8 shown;
[0103] Flow rate is set to be 0.9mL / min and 1.1mL / min in the reversed-phase high-performance liquid chromatography condition respectively, and other conditions are consistent with embodiment 1, and system suitability solution is carried out reversed-phase high-performance liquid chromatography detection, and gained spectrogram is as follows Figure 9 ~ Figure 10 shown;
[0104] The temperature of the chromatographic column in the reversed-phase high-performance liquid chromatogra...
Embodiment 3
[0108] Solution stability testing:
[0109] The preparation method of need testing solution, 1% self control solution and system suitability solution is identical with embodiment 1, respectively the 0h, 2h, 4h, 8h, 12h, 16h, 24h, 32h and 48h to system suitability solution Reverse-phase high-performance liquid chromatography detection; respectively at 0h, 3h, 7h, 11h, 15h, 23h and 33h for reverse-phase high-performance liquid chromatography detection of the test solution; respectively at 0h, 3h, 7h, 11h, 15h , 23h and 38h carry out reversed-phase high-performance liquid chromatography to 1% self control solution and detect detection condition and embodiment 1 identically, the obtained result table 3~shown in table 5:
[0110] Table 3 System Suitability Solution Stability Test Results
[0111] point in time EP-A EP-B EP-D EP-C YGT EP-E EP-F M1 EP-G 0h 100.00% 100.00% 100.00% 100.00% 100.00% 100.00% 100.00% 100.00% 100.00% 2h 99.93% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com