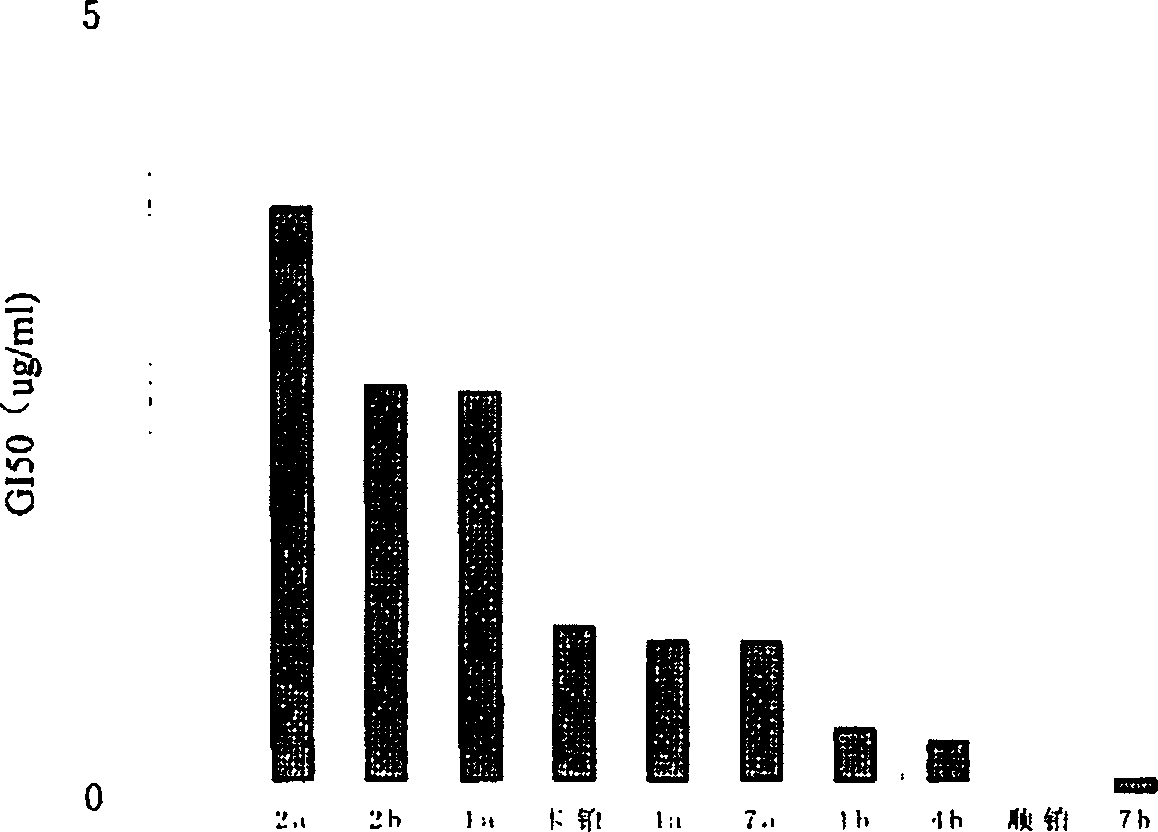
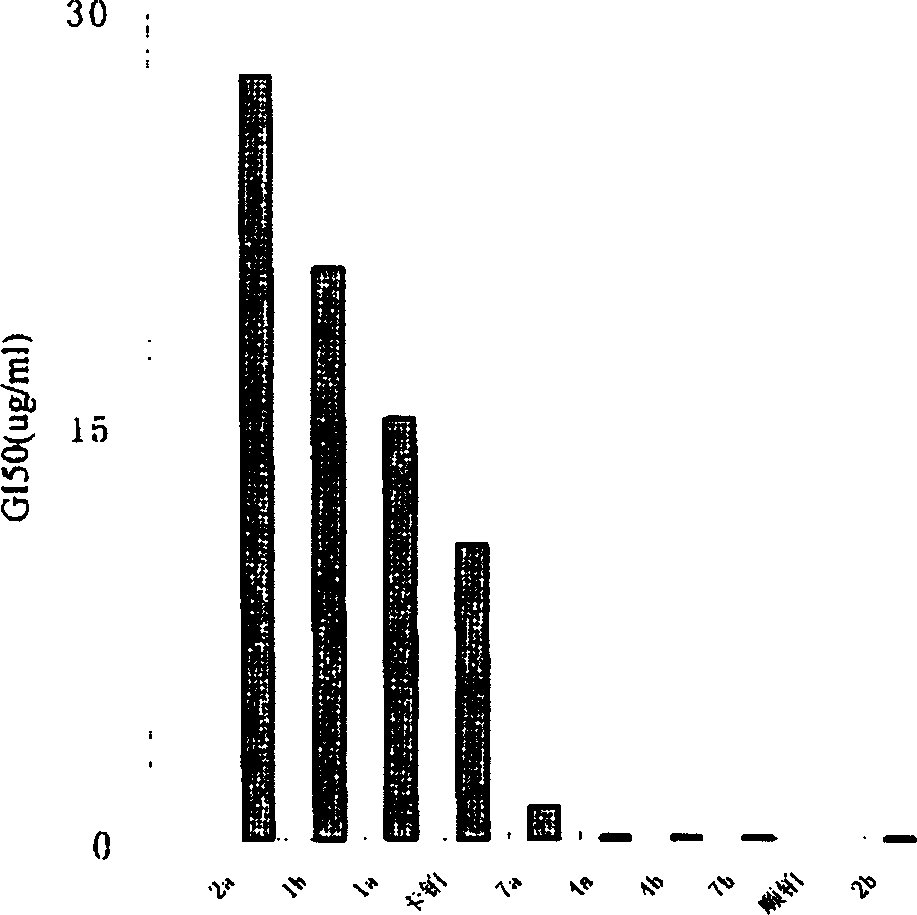
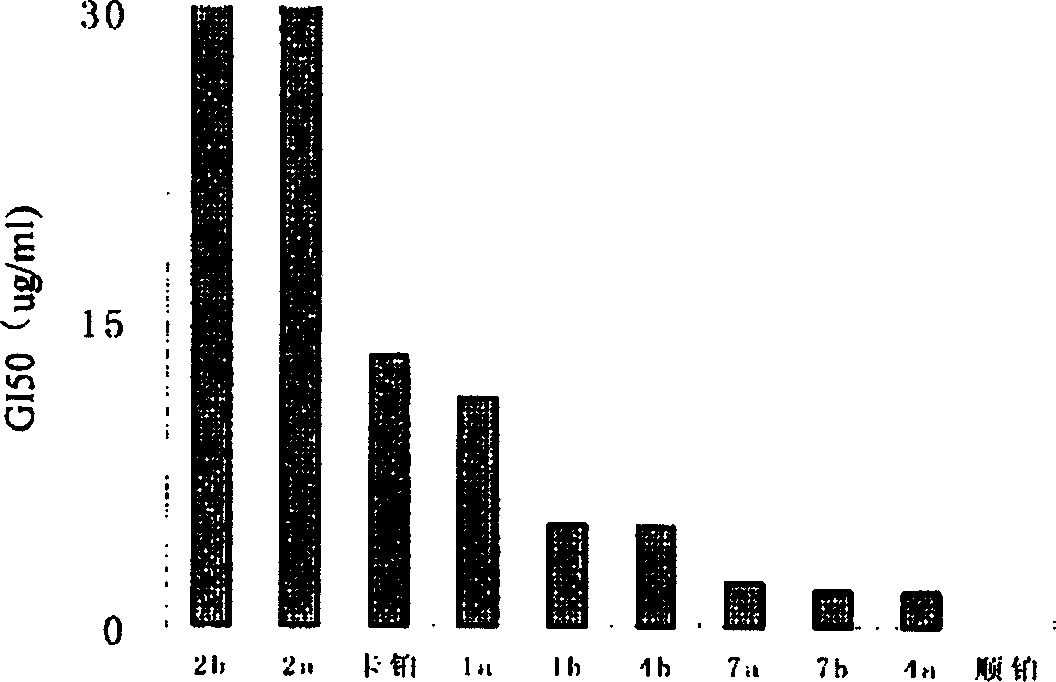
Antitumor platinum (II) compound using camphor acid radical as ligand
A technology of camphoric acid and complexes, applied in the field of new platinum complexes, can solve the problems of compound inactivity and increased toxicity of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Synthesis of cis-(DL-camphorate) diammine platinum (II)
[0032] cis-diiododiammine platinum (II) (0.97 g, 2 mmol) and silver nitrate (0.68 g, 4 mmol) were mixed and added to water (50 ml), and reacted at 60° C. for 24 hours in the dark and nitrogen. Diatomaceous earth assisted filtration, the aqueous solution of DL-camphoric acid (0.40 g, 4 mmol) and sodium hydroxide (0.16 g, 4 mmol) was added to the filtrate, and the reaction was carried out under nitrogen at 60 ° C for 16 hours in the dark, and the solution was concentrated , a large amount of white solid was precipitated. Filter, wash repeatedly with water, ethanol and ether, and dry under vacuum at 60°C to obtain 0.41 g (48%) of the product.
[0033] IR(KBr): 3424vs(br), 3266vs(br), 2967m, 2881w, 1594vs, 1544vs, 1460m, 1384vs, 1355vs
[0034] 1 H-NMR (D 2 O / TMS): δ0.47-0.67(m, 3H), 0.91-1.08(m, 6H), 1.24-1.31(s, 1H), 1.60(s, 1H), 1.74-1.75(s, 1H), 2.08(m, 1H), 2.55-2.56(s, 1H)
[0035] ESI-MS: [...
Embodiment 2
[0036] Example 2: Synthesis of cis-(DL-camphorate) two (isopropylamine) platinum (II)
[0037] cis-diiodo·bis(isopropylamine)platinum(II) (1.13 g, 2 mmol), silver nitrate (0.68 g, 4 mmol) were mixed and added to water (50 ml), and reacted in the dark at 60°C under nitrogen gas After 24 hours, diatomaceous earth was assisted to filter, and the aqueous solution of DL-camphoric acid (0.40 g, 4 mmol) and sodium hydroxide (0.16 g, 4 mmol) was added to the filtrate, and it was reacted in the dark at 60° C. for 16 hours, The solution was concentrated and a large amount of white solid precipitated out. It was filtered, washed repeatedly with water, ethanol and ether, and dried under vacuum at 60°C to obtain 0.39 g (38%) of the product.
[0038] IR(KBr): 3430s(br), 3217s(sh,br), 2971vs, 2880w, 1595vs(br), 1462m, 1383vs, 1351vs, 1164w, 1118w
[0039] 1 H-NMR (DMSO-d 6 / TMS): δ0.70-0.85 (m, 3H), 0.97-1.26 (m, 18H), 1.44-2.36 (br, 4H), 2.64-2.70 (br, 1H, CH), 2.96-3.18 (m, 2H), 5.85-...
Embodiment 3
[0041] Example 3: Synthesis of cis-(D-camphorate)·[(1R,2R)-1,2-trans-cyclohexanediamine] platinum(II)
[0042]Cis-dichloro[(1R, 2R)-1,2-trans-cyclohexanediamine] platinum (II) (0.76 g, 2 mmol), silver nitrate (0.68 g, 4 mmol) were mixed and added to water ( 50 milliliters), under 60 ℃, shielded from light and reacted with nitrogen for 24 hours, diatomaceous earth assisted filtration, added the aqueous solution of D-camphoric acid (0.40 gram, 4 mmol) and sodium hydroxide (0.16 gram, 4 mmol) in the filtrate , React at 60°C for 16 hours in the dark and pass through nitrogen, the solution is concentrated, and a large amount of white solid is precipitated. Filter, wash repeatedly with water, ethanol and ether, and dry under vacuum at 60°C to obtain 0.65 g (64%) of the product.
[0043] IR(KBr): 3424vs(br), 3226s(sh,br), 2939s, 2872w, 1598vs(br), 1457m, 1381vs, 1350s, 1169w, 1126w, 1063w
[0044] 1 H-NMR (DMSO-d 6 / TMS): δ0.84-0.91(m, 3H), 1.09-1.20(m, 6H), 1.28(m, 4H), 1.48(m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com