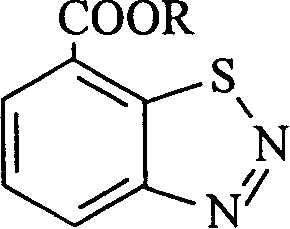
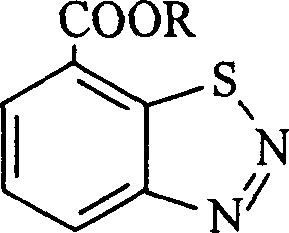
Benzothiadiazole compound and application in plant cell thereof
A technology for benzothiadiazoles and compounds is applied in the field of benzothiadiazole compounds, which can solve the problems of lack of resources, slow growth of Taxus chinensis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Synthesis of 7-carboxybenzo-1,2,3-thiadiazole trifluoroethyl ester (compound 1):
[0018] Mix 7-carboxybenzothiadiazole (3.3mmol) and 8ml thionyl chloride, add a drying tube and a tail gas absorption device, heat the oil bath to 80-100°C, and keep it for 7-8 hours. After cooling to room temperature, the unreacted thionyl chloride was distilled off under reduced pressure to obtain a somewhat sticky solid, which was dissolved in toluene for further use. Measure 4ml of trifluoroethanol and 4ml of dry toluene and mix them in a small flask, add triethylamine, slowly add acid chloride toluene solution dropwise under stirring. Stir at room temperature for 2-4 hours. The reaction mixture was poured into ice water, extracted with ethyl acetate and washed with NaHCO 3 Wash once with saturated solution, twice with water, anhydrous MgSO 4 dry. Filter and concentrate to obtain a yellow-brown solid, which is separated by 300-400 mesh silica gel column chromatography. The obtaine...
Embodiment 2
[0022] Synthesis of benzo-1,2,3-thiadiazole-7-carboxylic acid-2,2,3,3,3-pentafluoropropyl ester (compound 2):
[0023] The acid chloride prepared by 7-carboxybenzothiadiazole (2.2mmol) (the preparation of the acid chloride is the same as in Example 1) was dissolved in toluene, and was added dropwise to a solution composed of pentafluoropropanol, toluene and triethylamine under stirring , the dropwise addition time is about 1 hour. The reaction was continued at room temperature for 5 hours. Pour the reaction mixture into water, extract with ethyl acetate, dilute K 2 CO 3 Solution washing, water washing, anhydrous MgSO 4 dry. Filtrate and concentrate to obtain a brown solution, which was separated by 300-400 mesh silica gel column chromatography to obtain 0.23 g of light yellow prismatic crystals, with a yield of 33.5% and a melting point of 79-80°C.
[0024] 1 HNMR (SF: 500MHz, DMSO-d 6 )δ (ppm): 9.10-9.12 (d, 1H, J = 8.12Hz), 8.44-8.45 (d, 1H, J = 7.32Hz), 7.98-8.01 (t,...
Embodiment 3
[0026] Synthesis of benzo-1,2,3-thiadiazole-7-carboxylic acid-pentafluorobenzyl ester (compound 3)
[0027] Other reaction conditions are the same as in Example 1 except pentafluorobenzyl alcohol. Obtained 0.42 g of pale yellow needle-like crystals with a yield of 53.0% and a melting point of 146-7°C.
[0028] 1 HNMR (SF: 500MHz, DMSO-d 6 )δ (ppm): 9.03-9.05 (d, 1H, J = 8.10Hz), 8.39-8.41 (d, 1H, J = 7.35Hz), 7.91-7.94 (t, 1H, J 1 =7.65Hz,J 2 =7.83Hz), 5.62(s, 2H). HRMS, M + (m / e) 359.9983 (calc. 369.9992).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com