Therapeutic perparation for inhalation containing parathyroid hormone, PTH
A parathyroid hormone and therapeutic agent technology, applied to medical preparations containing active ingredients, medical preparations with non-active ingredients, metabolic diseases, etc., can solve problems such as platform phenomena
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1.1 Therapeutic Agents of PTH 1-84 for Inhalation
[0035] Prepare an aqueous solution of the following composition:
[0036] Human PTH 1-84 41mg
[0037] Citric acid, monohydrate 57mg
[0038] Sodium citrate 113mg
[0039] Lactose 3888mg
[0040] Water about 53ml
[0041] Adjust the pH to 5.0. The solution was concentrated by evaporation for about one day at 37°C. The resulting solid cake was crushed and passed through a 0.5 mm sieve, and the resulting powder was micronized by a jet mill to particles of 2 microns in diameter.
[0042] 1.2 Therapeutic Agents of PTH 1-34 for Inhalation
[0043] Prepare an aqueous solution with the following composition:
[0044] Human PTH 1-34 11.2mg
[0045] Citric acid, monohydrate 66mg
[0046] Sodium citrate 131mg
[0047] Lactose 4589mg
[0048] Water about 52ml
[0049] This solution was worked up as described above in Example 1.1.
[0050] 1.3 Therapeutic PTH preparations containing enhancers
[0051] Prepare an aqu...
Embodiment 2
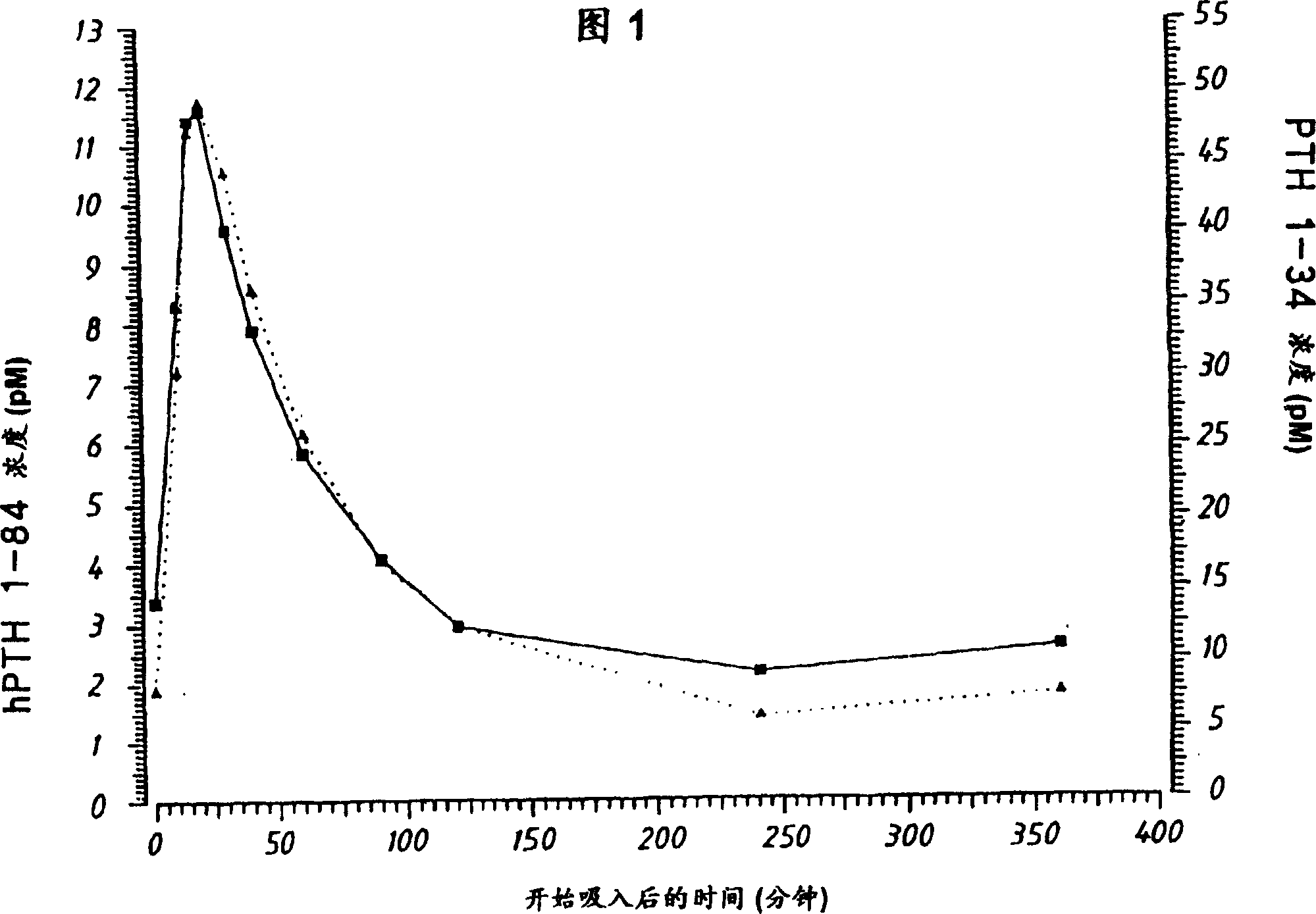
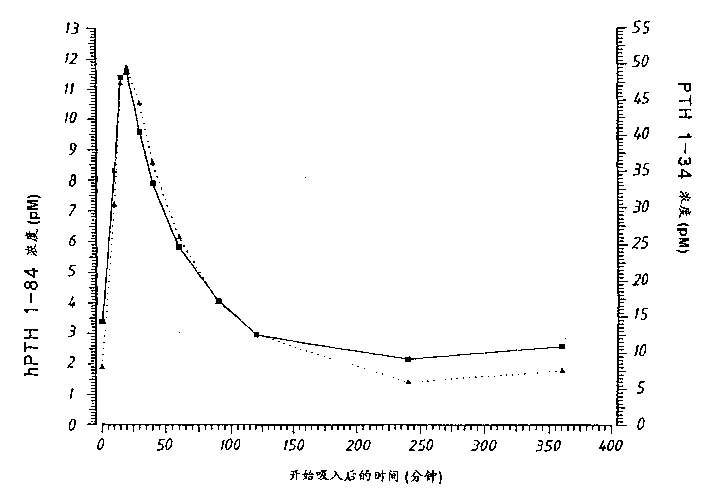
[0060] Pharmacokinetic experiment
[0061] 2.1 Powder formulations and inhalation systems
[0062] Human PTH 1-84 or PTH 1-34 were prepared according to Examples 1.1 and 1.3, respectively. The powder formulation was compressed in a dust container and continuously produced as a dry powder aerosol by a Wright Dust Feed (WDF). This aerosol is generated by wiping the tablet in a dust collector. The material flow rate through the WDF was 8.0 liters / minute.
[0063] Inhaled dose (ID) was determined by measuring tidal volume (ITV) and PTH concentration during inhalation.
[0064] 2.2 Treatment
[0065] English Beagle dogs (n=5, each formulation) were fasted for 16 hours prior to inhalation, and experiments were performed in the morning. with Plegecil and Penthotal Dogs were anesthetized, intubated and exposed to PTH1-34 or PTH1-84 for approximately 10 minutes.
[0066] PTH concentrations were determined in venous blood samples collected from the jugular vein into heparini...
Embodiment 3
[0069] bone effect
[0070] Bone response was measured as the mineral density (weight / volume) of the distal femur in ovariectomized osteoporotic rats after 4 weeks of dosing (dosing started 6 weeks after ovariectomy). The results obtained indicate that inhalation of full-length PTH has a significant effect on femur formation.
[0071] Table 1
[0072]
[0073] S.E. = standard error of the mean
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com