Benzopyran-containing compounds and method for their use
A technology of compounds and compositions, applied in the treatment of estrogen-sensitive diseases, anti-estrogen compounds, stereoselective substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] 7-Hydroxy-3-(4′-hydroxyphenyl)-4-methyl-2-(4″-(2-piperidinoethoxy)phenyl)-2H-benzopyran (EM -343) Synthesis Synthesis A (this synthesis is described in Scheme 1 below)
[0109] Process 1
[0110]
[0111] The foregoing synthesis was carried out as follows:
[0112] Triphenol 3
[0113] Resorcinol 1 (89.2 mg.0.810 mol) and acid 2 (135.4 mg, 0.890 mol) (both compounds were purchased from Aldrich Chemical Co., Milwaukee, Wis.) in boron trifluoride etherate ( 300ml) and toluene <240ml) were heated at 100°C for 3 hours and then cooled to room temperature. The resulting suspension was stirred overnight with 12% aqueous sodium acetate (400ml). The resulting precipitate was filtered off and washed with distilled water (2 x 1 L) and 12% aqueous sodium acetate (400 ml). The solid was then stirred overnight with 12% aqueous sodium acetate (1.2 L). The precipitate was filtered off, washed with distilled water (500ml) and recrystallized (ethanol:wate...
Embodiment 2
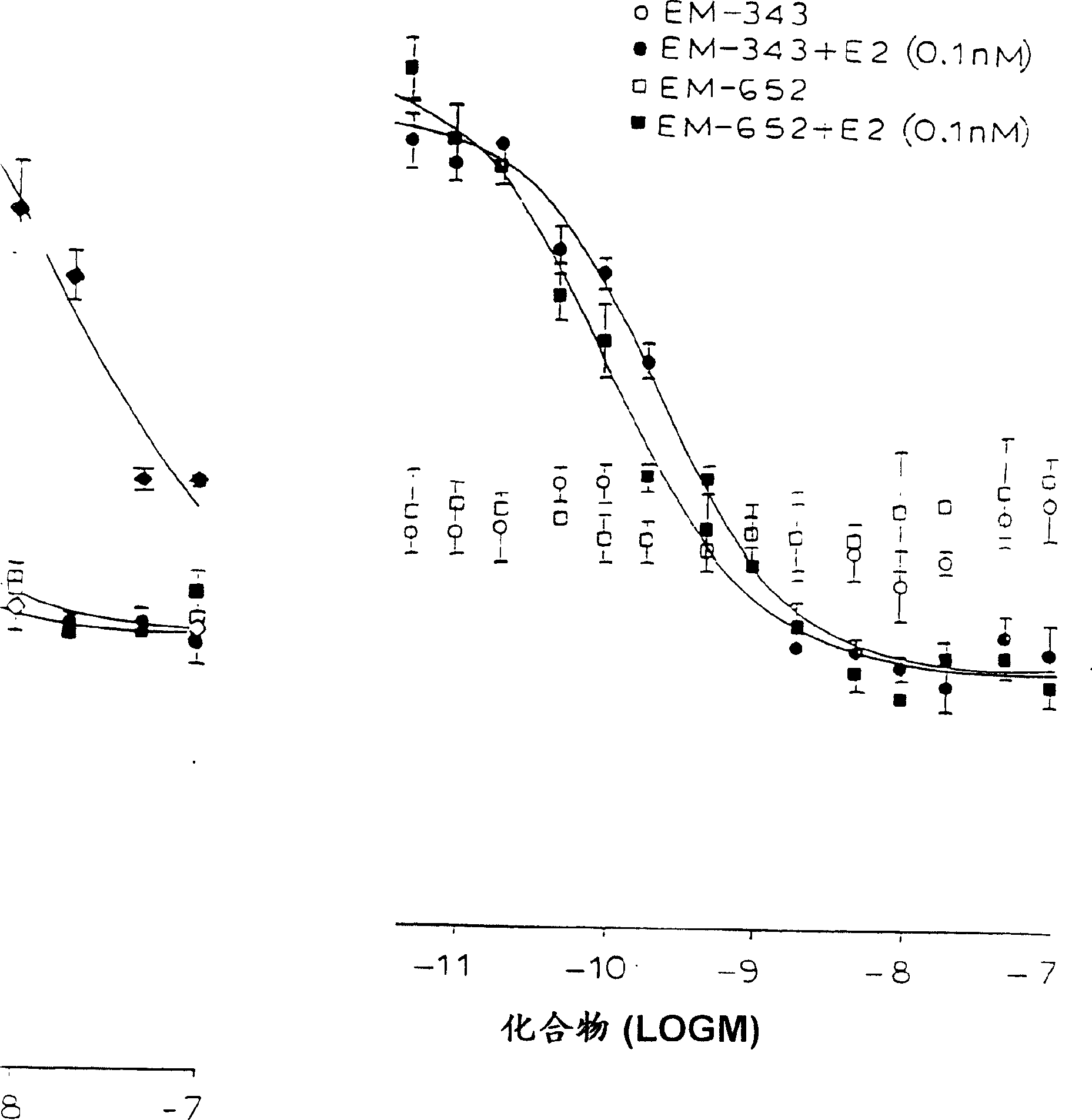
[0133] Separation of (+)-7-hydroxy-3-(4′-hydroxyphenyl)-4-methyl-2-(4″-(2-piperidinoethoxy)phenyl)-2H-benzene Pyran (EM-652)
[0134] Separation of the enantiomers of EM-343 (209 g) (see scheme 4 below) was carried out at room temperature using a 10 x 50 cm Daicel Chiralpark ADTM columns (available from Chiral Technologies, Inc. Extons, P.A.) were performed several times. The eluent is hexane / ethanol / diethylamine: 80 / 20 / 0.02 (volume ratio). The final product was evaporated to dryness under vacuum at 40°C. At room temperature, using Daicel Chiralpark AD YM Column (purchased from Chiral Technologies, Inc. Extons, P.A.), enantiomeric purity was checked by analytical HPLC, UV detection: 254 nm. The eluent is hexane / ethanol / diethylamine: 80 / 20 / 0.02, the flow rate is 1.0ml / min., and the following sequence of eluents is obtained:
[0135] Fraction 1 (the first eluting fraction)
[0136] (+)-7-Hydroxy-3-(4′-hydroxyphenyl)-4-methyl-2-(4″-(2-piperidinoethoxy)phenyl)-2H-benz...
Embodiment 3
[0144] Chemical Resolution Separation of Enantiomers of EM-343
[0145] To a solution of EM-343 (918 mg, 2.00 mmol) in methanol (5 ml) was added a solution of (1S)-(+)-10-camphorsulfonic acid (466 mg, 2.00 mmol) in methanol (20 mL). The resulting solution was left at room temperature for one day and then at -20°C for two days. Scrape occasionally to aid in crystallization. The crystals were filtered off, washed with a minimum amount of methanol, dried and determined for optical rotation ([α] D 25 +41°, methanol), yielding 507 mg of the salt. If necessary, the crystals were recrystallized once or twice with a minimal amount of hot methanol under the same conditions as above to obtain 100 mg of the salt ([α] D 25 +99°, methanol). The mother liquor additionally gave 129 mg of the salt ([α] D 25 +115°).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com