Compound lidocaine microneedle patch for easing pain
A technology of compound lidocaine and lidocaine hydrochloride, which is applied in the direction of tablet delivery, pharmaceutical formulations, and medical devices, to achieve the effects of improving stability, improving curative effect, and improving release and absorption in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The second aspect of the present invention provides the preparation method of the above-mentioned compound lidocaine / prilocaine microneedle patch, which comprises the following steps:
[0025] (1) lidocaine hydrochloride and prilocaine hydrochloride of the recipe quantity are added in an appropriate amount of water to be fully dissolved to obtain an aqueous drug solution;
[0026] (2) the host material of recipe quantity is added in the medicine aqueous solution that step (1) obtains, and stirring obtains homogeneous solution;
[0027] (3) inject the solution obtained in step (2) into a PDMS mold after swelling for 2-6 hours, centrifuge at low temperature, and dry;
[0028] (4) Pour the fully swollen backing material aqueous solution in the prescribed amount into the needle tip layer obtained in step (3), centrifuge at low temperature, dry, and demould, that is, it is obtained.
[0029] Preferably, the solution swelling time of the step (3) is 4 hours.
[0030] Prefer...
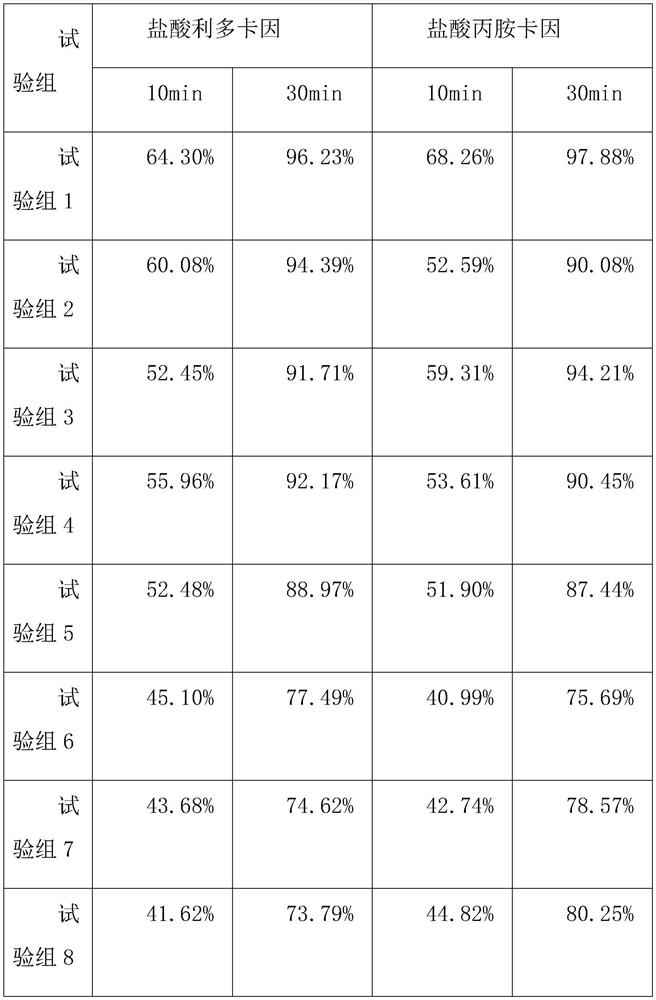
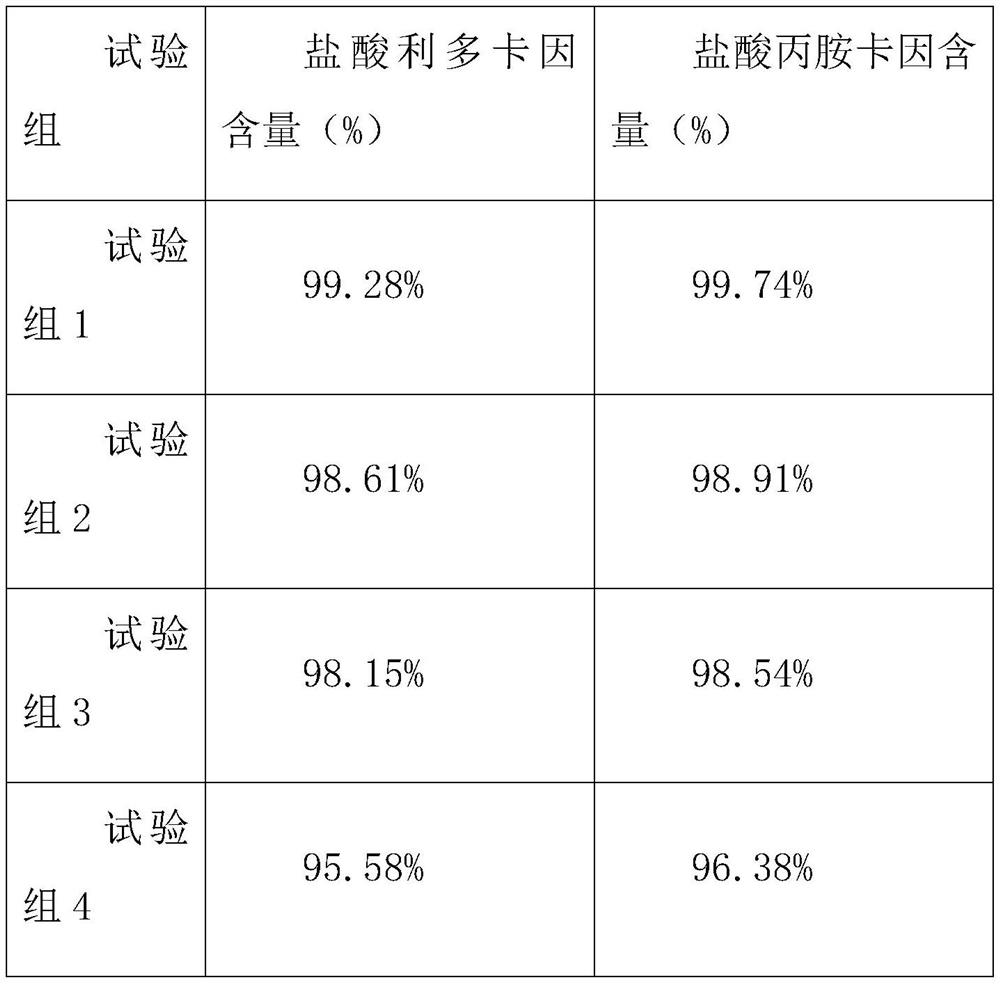
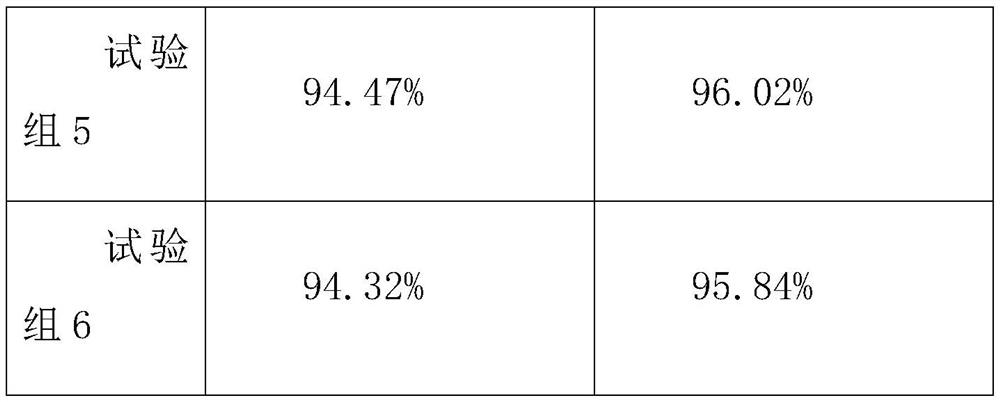
Embodiment 1
[0038] Embodiment 1 Compound lidocaine / prilocaine microneedle patch MN1 of the present invention
[0039] Weigh 0.015g lidocaine hydrochloride and 0.01g prilocaine hydrochloride, add 2.5mL purified water, and fully dissolve to obtain an aqueous drug solution; 0.19g hyaluronic acid (relative molecular mass 1*104-1*105), 0.19 g chondroitin sulfate and 0.095g PVP K30 were added to the above-mentioned drug aqueous solution in turn, and a homogeneous solution was obtained by stirring; after the above-mentioned solution was swollen for 4 hours, 60 μL was injected into the PDMS mold, and centrifuged at 4°C, 4000 r / min for 10 minutes to make the solution fully Fill the needle-shaped cavity of the mold; after centrifugation and drying at 25 °C for 1 hour, add 100 μL of the fully swollen 0.2 g / mL polyvinyl alcohol aqueous solution backing material, and centrifuge under the same conditions to remove the air bubbles in the backing solution. After drying at 25° C. for 12 hours, the mold is...
Embodiment 2
[0040] Embodiment 2 Compound lidocaine / prilocaine microneedle patch MN2 of the present invention
[0041] Adjust the ratio of matrix materials, and replace the composition of 0.19g hyaluronic acid, 0.19g chondroitin sulfate and 0.095g PVPK30 in Example 1 with the composition of 0.095g hyaluronic acid, 0.19g chondroitin sulfate and 0.19g PVP K30 , and the rest of the prescription and preparation process are the same as in Example 1, to obtain compound lidocaine / prilocaine microneedle patch MN2 of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com