Benzophenone dimer compound and application thereof
A compound and application technology, which is applied in the field of benzophenone dimer compounds, can solve problems such as brown adipose tissue abnormalities, achieve good tyrosinase inhibitory activity, treat obesity, and have good market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
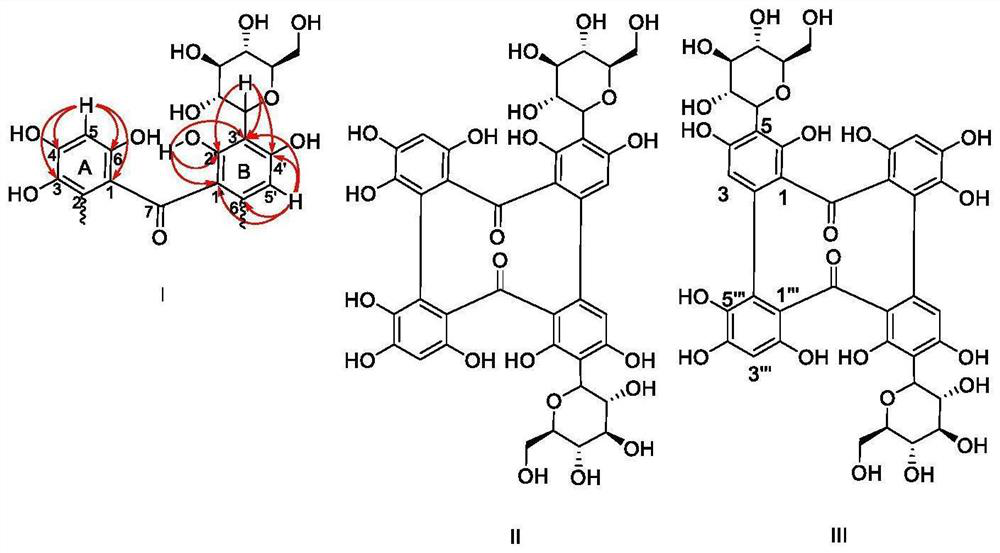
[0052] The total extract was prepared by extracting the leaves of C. chinensis with pure water. After removing sugar by macroporous resin, the water-soluble chemical constituents of C. chinensis were separated by normal and reversed-phase silica gel chromatography. Combined with one-dimensional and two-dimensional nuclear magnetic resonance spectroscopy picture( 1 H-NMR, 13 C-NMR, HSQC, HMBC), mass spectrometry (MS), ultraviolet absorption spectroscopy (UV), etc. and literature data to identify the three-dimensional structure of the compound. details as follows.
[0053] Extraction and Separation:
[0054] The dried medicinal powder (5kg) was extracted with pure water at 85°C for three times (0.5 hours each time). The extracted water was mixed and concentrated under reduced pressure to obtain the total extract. The total extract was passed through a macroporous resin D-101 column for gradient elution with methanol / water (0:100, 20:80, 40:60, 60:40, 80:20, v / v), and methano...
Embodiment 2
[0063] Example 2 Lipid-lowering activity of Caenorhabditis elegans
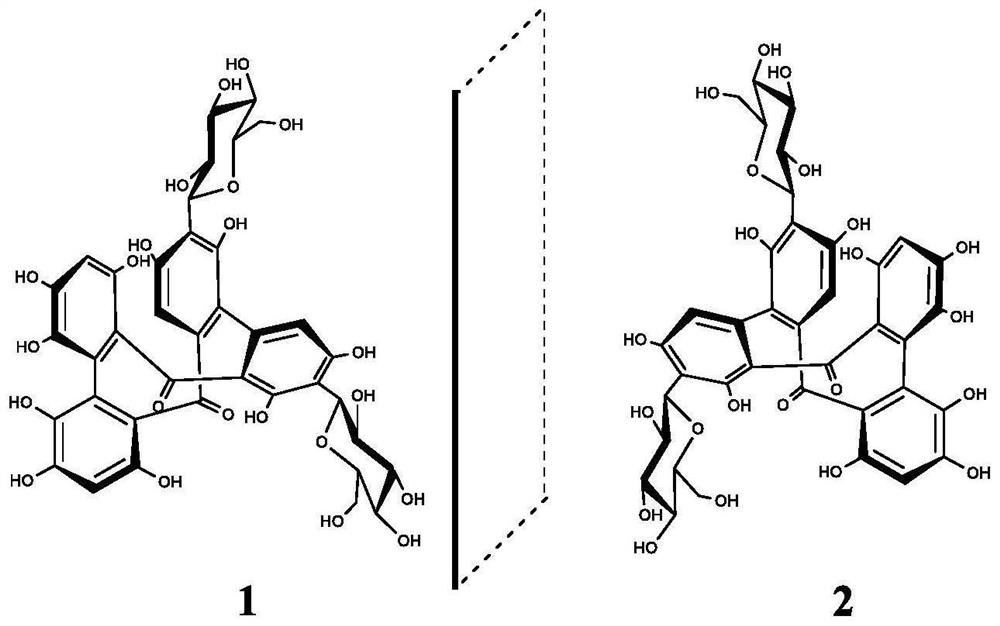
[0064] Firstly, C. elegans was treated with compounds 1 and 2 of the present invention, and oil red O and Sudan black B were used to stain and observe the nematodes, and the lipid-lowering activity of the monomer compounds was evaluated by the accumulation of fat in the body.
[0065] Coat 100 μL of the sample solution (final concentration of 100 μg / mL) and 100 μL of activated OP50 on NGM medium, place it in a 30°C incubator for 18 hours, and inoculate the nematodes at the L1 stage by contemporaneous culture. The nematodes were stained with Oil Red O and Sudan Black B after cultured at constant temperature (20°C) on drug-free NGM medium for 72h. Finally, the stained nematodes were washed with phosphate buffer, picked on a glass slide, and placed under a microscope to take pictures to observe the fat particles in the body. Orlistat and DMSO were positive and blank controls, respectively.
[0066] Obesity is ...
Embodiment 3
[0068] Example 3 Tyrosinase inhibitory activity
[0069] Using L-dopa as the substrate, the inhibition of tyrosinase activity was detected by a microplate reader. Compounds 1, 2 were first dissolved in DMSO and then diluted to a range of concentrations with phosphate buffered saline (PBS; pH=6.8). Simultaneously, levodopa and tyrosinase were dissolved in PBS. The concentrations of levodopa and tyrosinase were 0.5 mM and 0.06 mg / mL, respectively. Compound (50 μL), tyrosinase (50 μL) and PBS (50 μL) were mixed in a 96-well plate, reacted at 30° C. for 10 min, and then levodopa was added to the mixture and reacted at 30° C. for 5 min. Then, the absorbance of each solution was measured at 475 nm. The total solution system was 250 μL, and DMSO was used as the blank control. The tyrosinase inhibition rate was calculated as follows: Inhibition rate (%)=1-[(A 2 -A 1 ) / (B 2 -B 1 )]×100%
[0070] A 1 is the absorbance of the blank at 0 min, A 2 is the absorbance of 100 blank ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com