Preparation method of o-aryl benzamide compound
A technology for arylbenzamide and ketone compounds, which is applied in the field of preparation of o-arylbenzamide compounds, can solve the problems of inconvenient use and operation of organometallic reagents, achieve fewer reaction steps and reduce the loss of raw materials , the effect of wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] 3-Toluo[d][1,2,3]triazin-4(3H)-one (16.1 mg, 0.10 mmol), bromobenzene (21 μL, 0.2 mmol), nickel diethyl bromide in a glove box Glycol dimethyl ether (3.5mg, 0.001mmol), bipy2,2'-bipyridine (2.3mg, 0.0015mmol) and zinc powder (1.31g, 0.2mmol) were added to a microwave tube, followed by 0.2mL N,N - Dimethylacetamide, capped and removed from the glove box.
[0060] Then refluxed at 80°C for 12 hours, cooled to room temperature, opened the lid and added three drops of water to quench the reaction, removed the solvent under reduced pressure, and separated the crude product by column chromatography (petroleum ether:ethyl acetate=5:1) to obtain N-methyl-[1,1'-biphenyl]-2-carboxamide (18.4 mg, 87% yield).
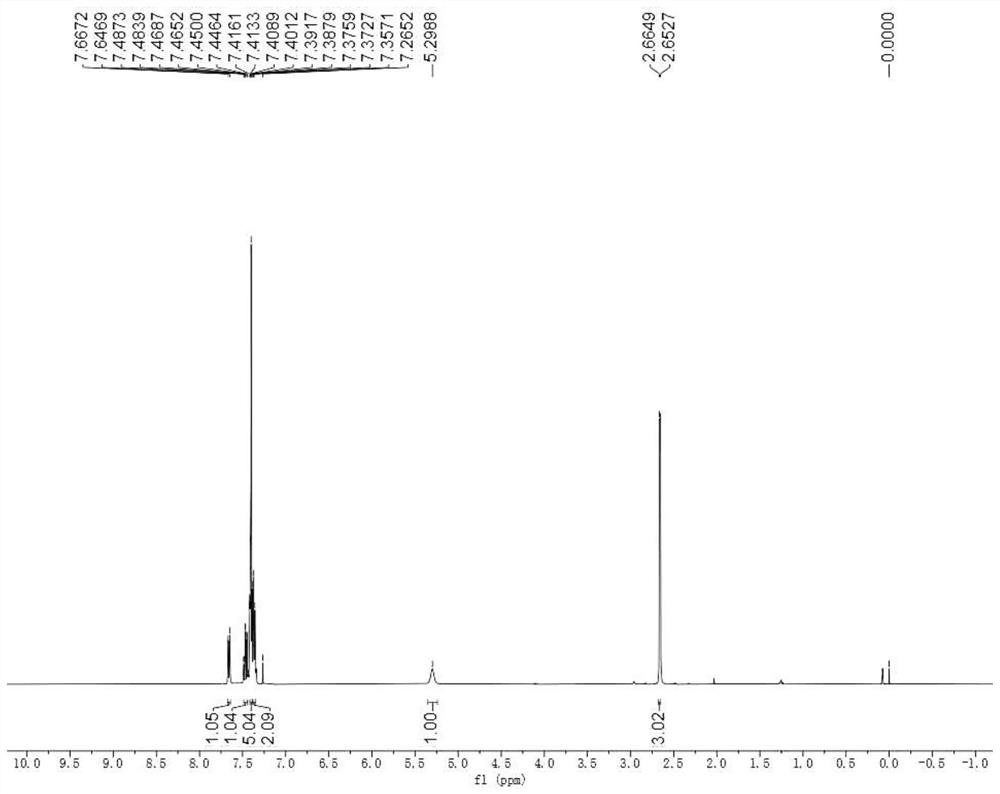
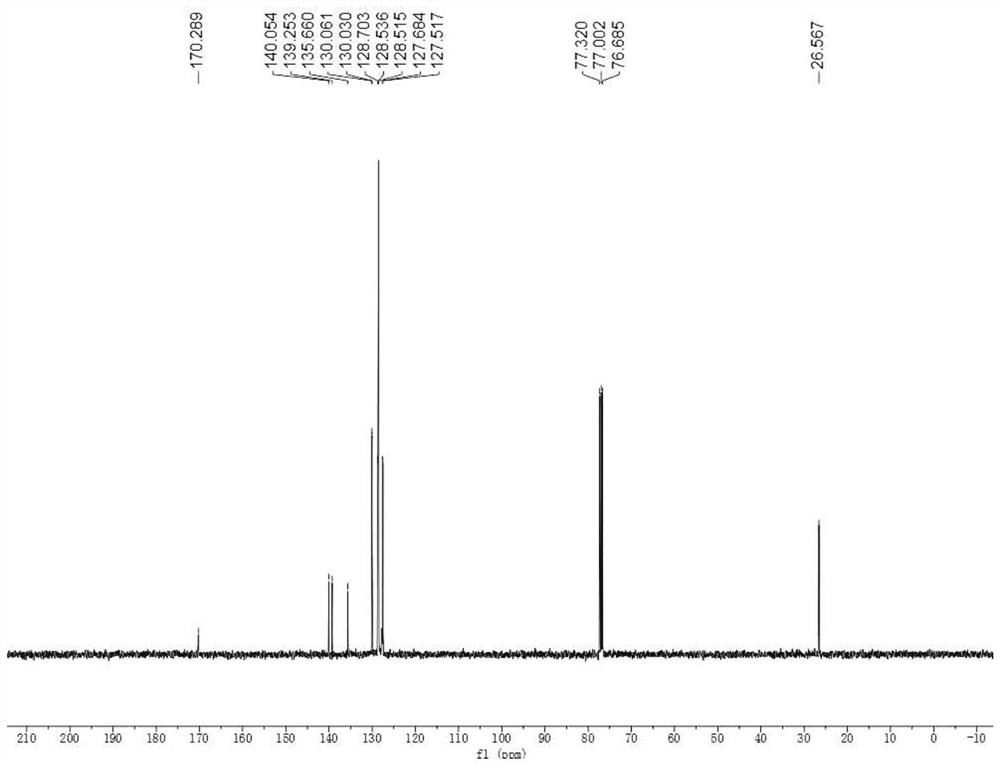
[0061] The hydrogen and carbon NMR spectra of the product are figure 1 and figure 2 , the spectral data is: 1 H NMR (400MHz, CDCl 3 )δ: 7.66(d, J=8.1Hz, 1H), 7.49-7.45(m, 1H), 7.42-7.36(m, 5H), 7.38-7.35(m, 2H), 5.30(s, 1H), 2.66 (d,J=4.9Hz,3H)ppm. 13 C{ 1 H}NMR (10...
Embodiment 2
[0063] In a glove box, combine 3-Toluo[d][1,2,3]triazin-4(3H)-one (16.1 mg, 0.10 mmol), 4-tert-butylbromobenzene (35 μL, 0.2 mmol), Nickel bromide diethylene glycol dimethyl ether (3.5 mg, 0.001 mmol), bipy (2.3 mg, 0.0015 mmol) and zinc powder (1.31 g, 0.2 mmol) were added to a microwave tube, followed by 0.2 mL of N,N-bismuth Methylacetamide, capped and removed from the glove box.
[0064] Then refluxed at 80°C for 12 hours, cooled to room temperature, opened the lid and added three drops of water to quench the reaction, removed the solvent under reduced pressure, and separated the crude product by column chromatography (petroleum ether:ethyl acetate=5:1) to obtain 4'-(tert-Butyl)-N-methyl-[1,1'-biphenyl]-2-carboxamide (24.1 mg, 90% yield).
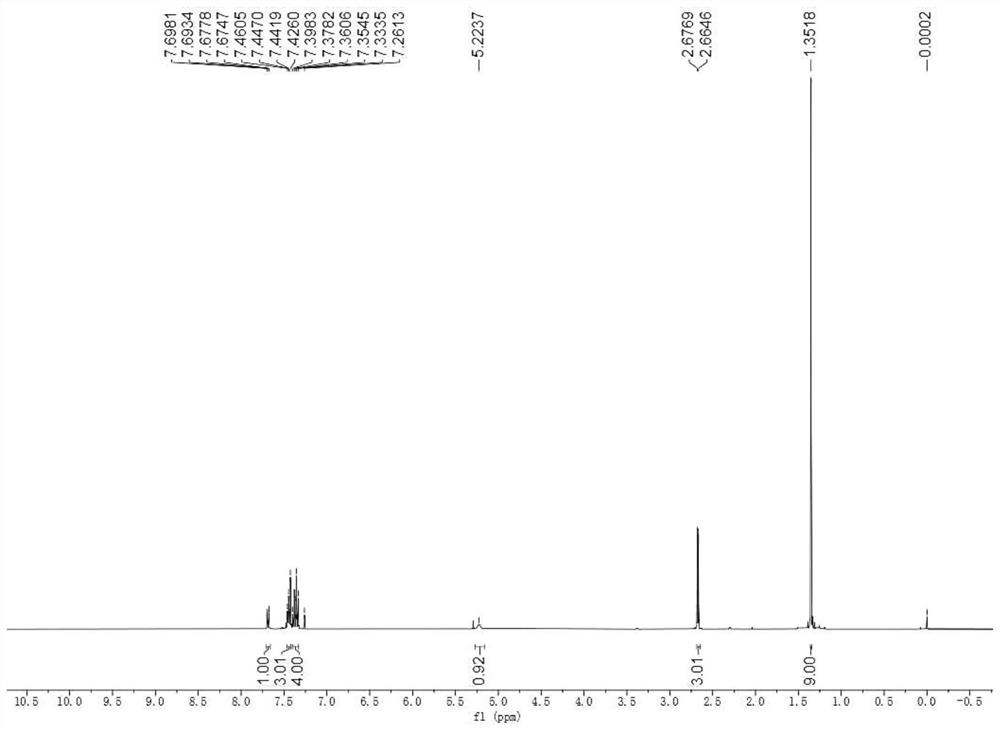
[0065] The hydrogen and carbon NMR spectra of the product are image 3 and Figure 4 , the spectral data is: 1 H NMR (400MHz, CDCl 3 )δ: 7.69-7.67(m, 1H), 7.47-7.42(m, 3H), 7.40-7.33(m, 4H), 5.22(s, 1H), 2.67(d, J=4.9Hz, 3H), 1.35 ...
Embodiment 3
[0067] In a glove box, combine 3-Toluo[d][1,2,3]triazin-4(3H)-one (16.1 mg, 0.10 mmol), 4-methoxybromobenzene (25 μL, 0.2 mmol), Nickel bromide diethylene glycol dimethyl ether (3.5 mg, 0.001 mmol), bipy (2.3 mg, 0.0015 mmol) and zinc powder (1.31 g, 0.2 mmol) were added to a microwave tube, followed by 0.2 mL of N,N-bismuth Methylacetamide, capped and removed from the glove box.
[0068] Then refluxed at 80°C for 12 hours, cooled to room temperature, opened the lid and added three drops of water to quench the reaction, removed the solvent under reduced pressure, and separated the crude product by column chromatography (petroleum ether:ethyl acetate=5:1) to obtain 4'-(Methoxy)-N-methyl-[1,1'-biphenyl]-2-carboxamide (22.0 mg, 91% yield).
[0069] The hydrogen and carbon NMR spectra of the product are Figure 5 and Image 6 , the spectral data are: 7.68–7.63(m,1H), 7.46–7.42(m,1H), 7.38–7.31(m,4H), 6.97–6.93(m,2H), 5.29(s,1H), 3.84 (s,3H),2.70(d,J=4.9Hz,3H)ppm. 13 C{ 1 H}N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com