Photosensitive monomer, photosensitive polymer based on photosensitive monomer, synthetic method and application
A technology of photosensitive polymer and synthesis method, which is applied in the field of stimuli-responsive carrier synthesis to achieve the effects of easy quality control, low synthesis cost, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
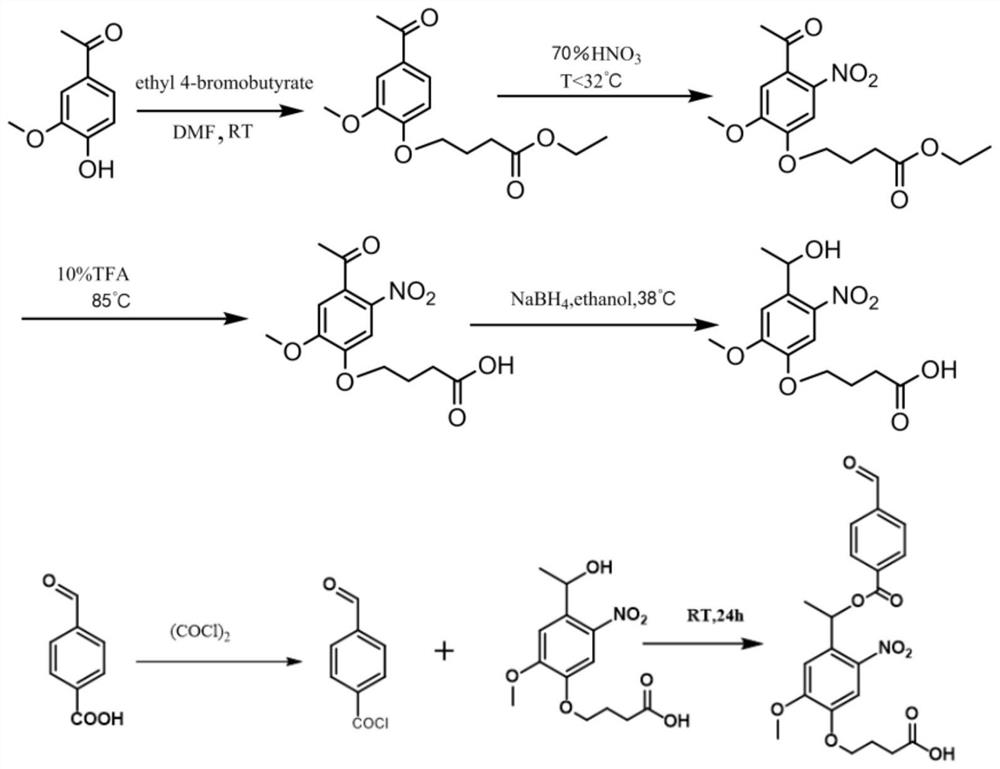
[0037] Its preparation method comprises the following steps:
[0038] 1) Alkylation reaction: Vanillyl ethyl ketone is dissolved in DMF, 4-bromobutyric acid ethyl ester is added after nitrogen purging for a period of time, potassium carbonate is added after nitrogen purging for a period of time, then water is added to stir, and vacuum-dried;
[0039] 2) nitration reaction: nitric acid is placed in an ice-water mixture to cool and slowly add acetic anhydride, then slowly add the alkylated product dissolved in acetic anhydride into the above-mentioned cold nitric acid solution dropwise, react in an ice-water bath, and in cold distilled water Carry out precipitation in, suction filtration, vacuum drying after washing, obtain nitration product;
[0040] 3) acidification reaction: react the nitrated product with an aqueous trifluoroacetic acid solution, filter after cooling, wash the solid residue, and vacuum dry to obtain the acidified product;
[0041] 4) Alcoholization reaction...
Embodiment 1
[0046] A kind of photosensitive monomer, its preparation method comprises the steps:
[0047] 1) Alkylation reaction: dissolve vanillyl ketone in DMF (N,N-dimethylformamide), purge with nitrogen for 10 min, add ethyl 4-bromobutyrate, vanilla ethyl ketone and 4-bromobutyric acid The molar ratio of ethyl ester was 1:1.5, 12.46 g of potassium carbonate was added to react for 24 hours after purging with nitrogen for 10 minutes, water was added and stirred for 2 hours, overnight at 4°C, suction filtration and vacuum drying at 40°C overnight.
[0048]2) Nitration reaction: place 70% nitric acid in an ice-water mixture and cool it to below 5°C, slowly add acetic anhydride in a ratio of 5:1, then slowly add the alkylated product dissolved in acetic anhydride to the above cold nitric acid solution dropwise , after reacting in an ice-water bath for 5 h, precipitation in cold distilled water at 4 °C overnight, suction filtration, and washing, and then vacuum drying at 40 °C overnight.
...
Embodiment 2
[0053] A kind of synthetic method of photosensitive polymer, this synthetic method is:
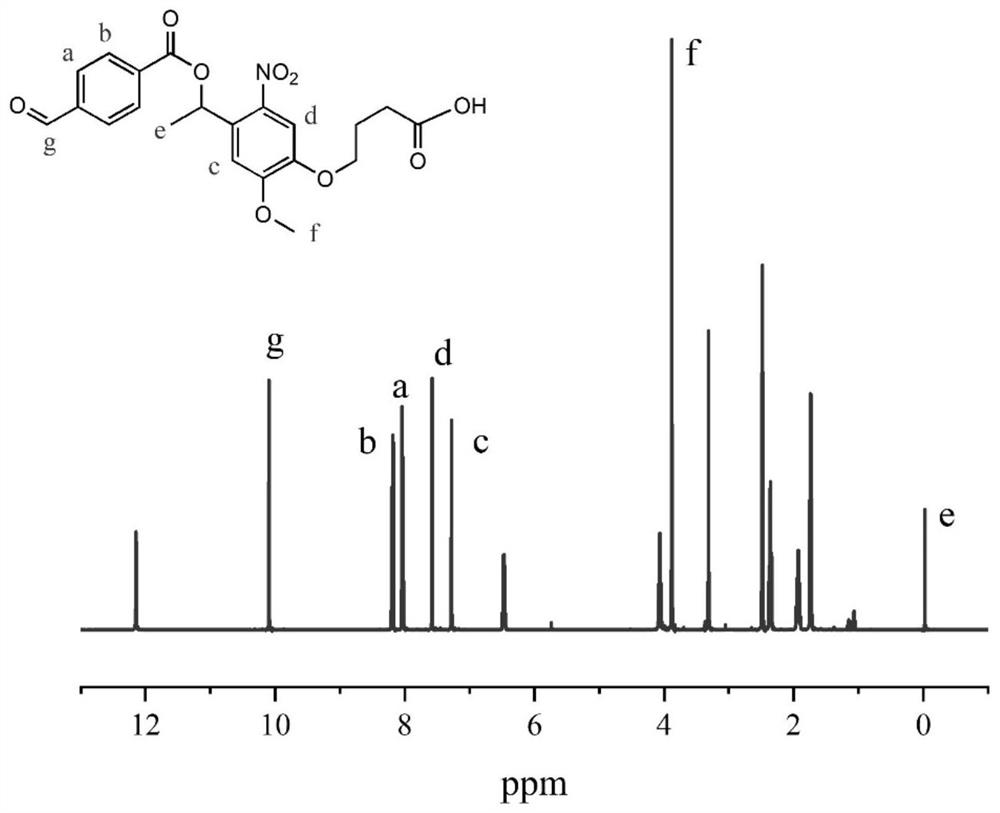
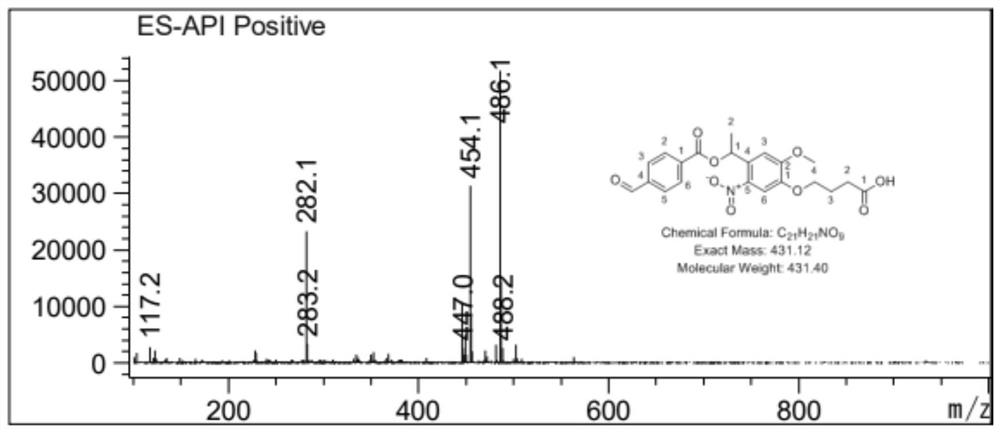
[0054] The synthesized 50 mg photosensitive monomer was dissolved in 10 mL DMSO with 70 mg EDC (1-ethyl-(3-dimethylaminopropyl) carbodiimide) and 25 mg NHS (N-hydroxythiosuccinimide) After mixing and stirring for half an hour, 50 mL of a 0.3 mg / mL chitosan solution dissolved in a 2.1% (w / v) aqueous acetic acid solution was added. The mixture was stirred at room temperature for 24 h, filtered, centrifuged at high speed, washed with deionized water, and freeze-dried. The particle size of the nano-drug carrier was measured after reconstitution with water, see Figure 5 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com