Drug loading system loaded with anti-cancer drug as well as preparation method and application of drug loading system
An anti-cancer drug and drug-carrying technology, which is applied in the direction of pharmaceutical formulations, anti-tumor drugs, drug combinations, etc., can solve problems such as narrow therapeutic index, organ intolerance to drugs, etc., to increase biodistribution, improve bioavailability, The effect of rapid mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0032] Preparation of empty neutral liposomes:
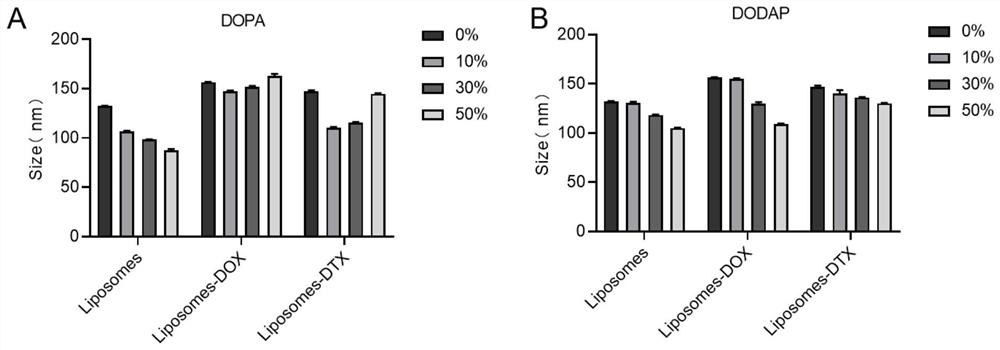
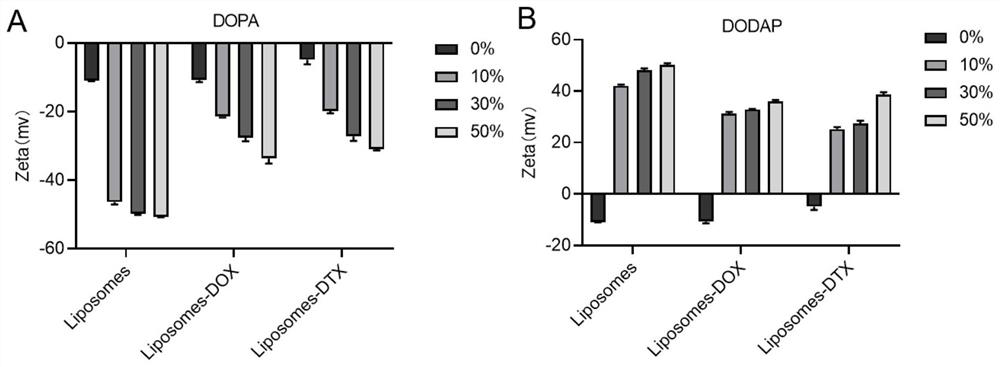
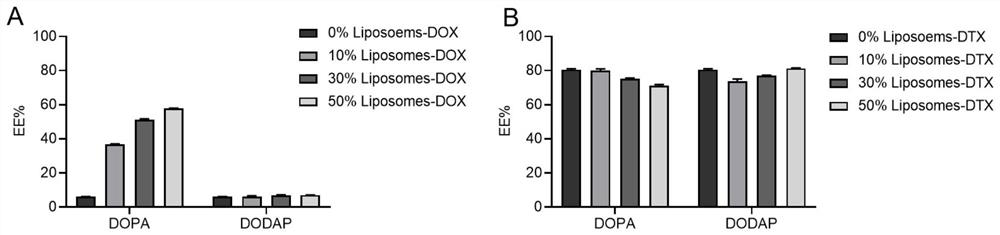
[0033] 2.57 mg (69 equiv.) 1,2-dioleoylglycerol-3-phosphoethanolamine (DOPE), 0.77 mg (40 equiv.) cholesterol (CHOL), 0.5 mg (4 equiv.) 1,2-dimyristoyl- sn-glycerol-3-methoxypolyethylene glycol (DMG-PEG) was dissolved in dichloromethane; 0 mg (0 equiv) of 1,2-dioleoyl-sn-glycerol-3-dissolved in dichloromethane was added Phosphate ester (DOPA, the two were mixed evenly, and the organic reagent was removed by rotary evaporation for 5 min to obtain a layer of phospholipid film; the obtained phospholipid film was hydrated with 4 ml of distilled water, ultrasonicated for 5 min, and the solution was transferred to a dialysis bag (MW=14000) , dialysis with pure water for 12h under stirring conditions, to obtain empty neutral liposome solution (0% Liposomes).
[0034] The particle size of the unloaded liposome solution is obtained by testing, and the results are as follows figure 1 As shown, the particle size of the liposome is betwee...
Embodiment 1-2
[0037] Preparation of empty 10% anionic liposomes and cationic liposomes:
[0038] (1) 2.22 mg (69 equiv.) 1,2-dioleoylglycerol-3-phosphoethanolamine (DOPE), 0.67 mg (40 equiv.) cholesterol (CHOL), 0.43 mg (4 equiv.) 1,2-dimeat Myristoyl-sn-glycero-3-methoxypolyethylene glycol (DMG-PEG) was dissolved in dichloromethane; 0.52 mg (12.6 equiv.) of 1,2-dioleoyl-sn-dissolved in dichloromethane was added Glycerol-3-phosphate (DOPA), the remaining steps were the same as those of Example 1-1 (except for transmission observation) to obtain an unloaded anionic liposome solution (10% DOPALiposomes).
[0039] (2) 2.32 mg (69 equiv.) 1,2-dioleoylglycerol-3-phosphoethanolamine (DOPE), 0.7 mg (40 equiv.) cholesterol (CHOL), 0.451 mg (4 equiv.) 1,2-dimeat Myristoyl-sn-glycero-3-methoxypolyethylene glycol (DMG-PEG) was dissolved in dichloromethane; 0.369 mg (12.6 equiv.) of 1,2-dioleoyl-3-dissolved in dichloromethane was added (Dimethylamino)propane (DODAP), the remaining steps were the same...
Embodiment 1-3
[0042] Preparation of empty 30% anionic liposomes and cationic liposomes:
[0043] (1) 2.22 mg (69 equiv.) 1,2-dioleoylglycerol-3-phosphoethanolamine (DOPE), 0.67 mg (40 equiv.) cholesterol (CHOL), 0.43 mg (4 equiv.) 1,2-dimeat Myristoyl-sn-glycero-3-methoxypolyethylene glycol (DMG-PEG) was dissolved in dichloromethane; 0.52 mg (12.6 equiv.) of 1,2-dioleoyl-sn-dissolved in dichloromethane was added Glycerol-3-phosphate (DOPA), the remaining steps were the same as those of Example 1-1 (except for transmission observation) to obtain an unloaded anionic liposome solution (10% DOPALiposomes).
[0044] (2) 2.32 mg (69 equiv.) 1,2-dioleoylglycerol-3-phosphoethanolamine (DOPE), 0.7 mg (40 equiv.) cholesterol (CHOL), 0.451 mg (4 equiv.) 1,2-dimeat Myristoyl-sn-glycero-3-methoxypolyethylene glycol (DMG-PEG) was dissolved in dichloromethane; 0.369 mg (12.6 equiv.) of 1,2-dioleoyl-3-dissolved in dichloromethane was added (Dimethylamino)propane (DODAP), the remaining steps were the same...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com