Preparation method of silodosin key intermediate
An intermediate and key technology, applied in the field of pharmaceutical synthesis, can solve problems such as large-scale production safety, product heavy metal residues, etc., and achieve the effects of time cost reduction, continuous operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
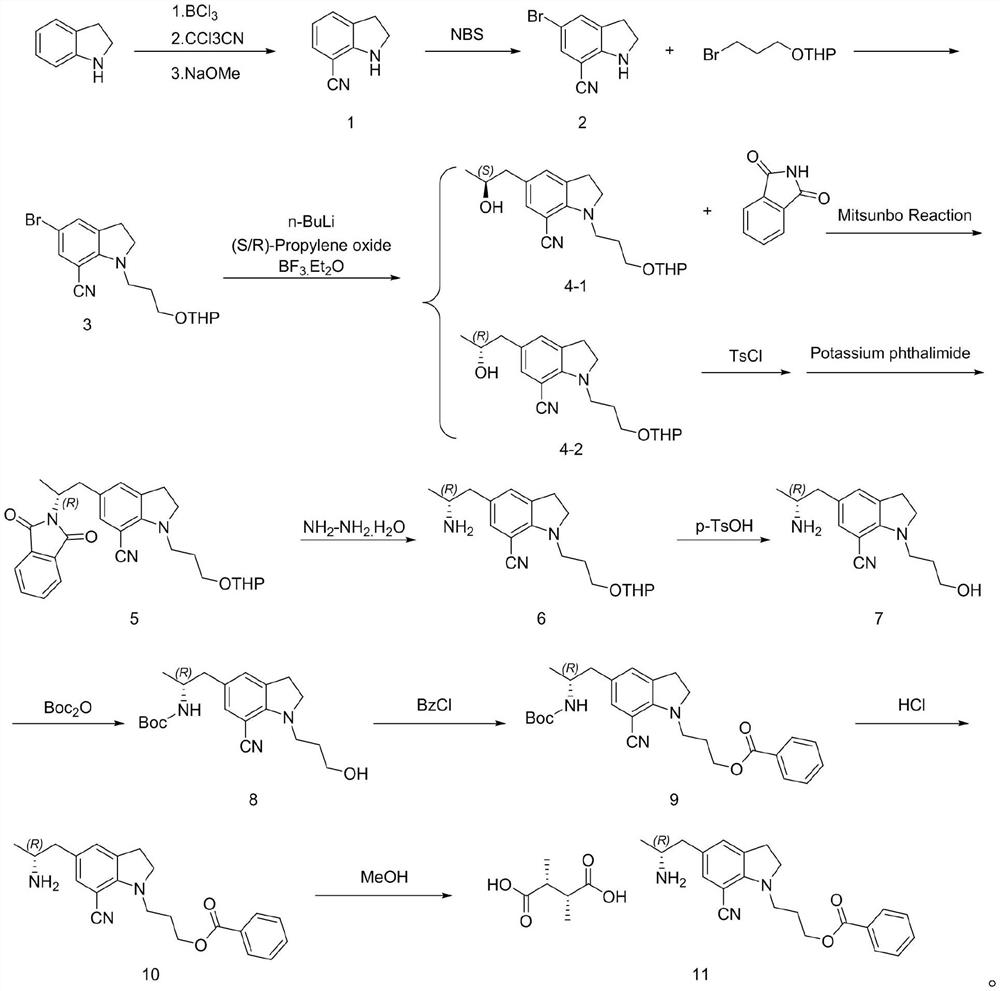
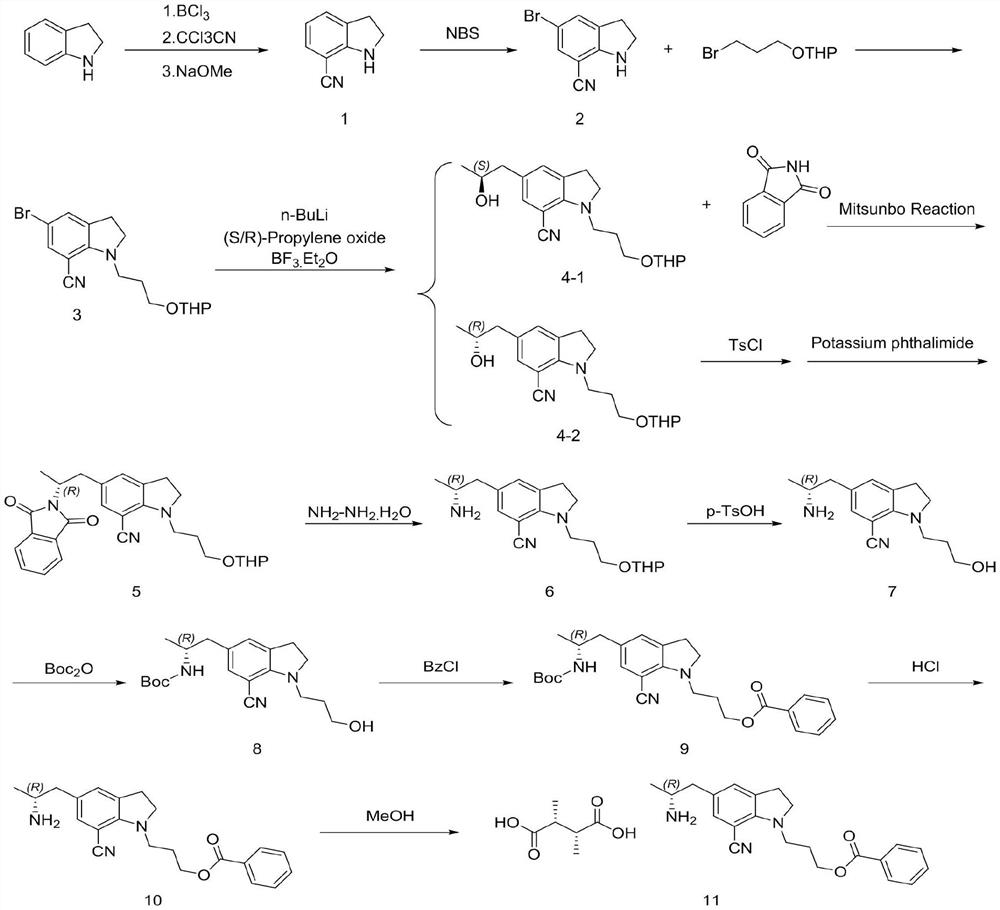
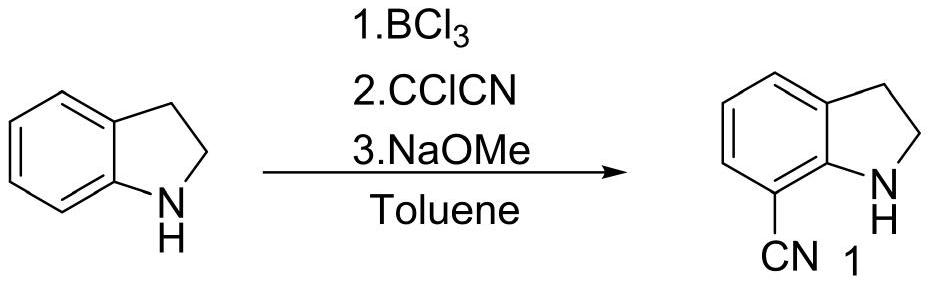
[0025] Example 1 The first step: the synthesis of 7-cyanoindoline
[0026]
[0027] In the 200L reactor, add 50L 1M BCl / toluene solution, dropwise add 20L toluene / 5.3Kg indoline solution, after mixing, heating to reflux for 1 hour; then cool to 60 ℃, slowly add 7.72Kg CCl CN, temperature control The reaction was stirred at 60 °C overnight; after the reaction was completed, the temperature was lowered to 10 °C, 30L methanol was slowly added to quench, and the reaction intermediate was obtained by filtration (this intermediate cannot be stored in the air for a long time, and the product is easily decomposed after moisture absorption. Filled with nitrogen and stored under vacuum), the intermediate and 150L of dichloromethane were added to a 500L reaction kettle, and 18Kg of 30% sodium methoxide solution was added to control the temperature not to exceed 25°C. After the reaction is complete, add 100 L of water, and separate layers at rest. The upper aqueous phase is washed ...
Embodiment 2
[0029] The second step: the synthesis of 5-bromo-7-cyano-1-(3-(propoxytetrahydropyran)indoline
[0030]
[0031]Into the 200L reaction kettle, add 3.82Kg of compound 1 and 50L of dichloromethane, add 4.95Kg of NBS in batches at 0-5°C, raise to room temperature and stir for 1 hour, add 40L of water to retain the organic phase. Dichloromethane was extracted, the organic phases were combined, and the organic phases were washed with 50 L of 5% aqueous sodium bicarbonate solution. The organic phase was distilled under reduced pressure to no flow, 45L of acetonitrile and 3.18Kg of sodium hydroxide were added, the temperature was raised to 45°C, and 20L of acetonitrile / 2-(3-bromopropoxy)tetrahydro-2H-pyran 6.71 was slowly added dropwise. Kg mixture was stirred and heated to reflux for 5 hours. After cooling to room temperature, saturated ammonium chloride solution was added to the reaction solution, concentrated under reduced pressure, extracted three times with ethyl acetate, an...
Embodiment 3
[0033] The second step: the synthesis of 5-bromo-7-cyano-1-(3-(propoxytetrahydropyran)indoline
[0034]
[0035] Into the 200L reaction kettle, 3.82Kg of compound 1 and 32L of acetonitrile were added, 4.95Kg of NBS was added in batches at 0-5°C, and then the reaction was stirred at room temperature for 1 hour. 40L of water was added, most of the acetonitrile was distilled off under reduced pressure, the temperature was lowered to 20-25°C, the filter cake was rinsed with water, and the organic phase was dried to no liquid. Then, 50L of acetonitrile and 12.82Kg of potassium carbonate were added and the temperature was raised to 45-55°C, 20L of acetonitrile / 2-(3-bromopropoxy)tetrahydro-2H-pyran 6.71Kg mixture was slowly added dropwise, and the temperature was stirred to reflux for 8 hours. . The temperature was lowered to room temperature, 60 L of water was added to the reaction solution, concentrated under reduced pressure, extracted three times with ethyl acetate, and the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com