Nanoformulations for topical and systemic fat reduction and uses thereof
A nano-preparation, systemic technology, applied in the direction of medical preparations and pharmaceutical formulations containing active ingredients, can solve the problem of safety and biocompatibility cannot be ignored, incompatible effects, gastrointestinal tract, liver and kidney damage. Large and other problems, to achieve the effect of inhibiting the occurrence and development of adipose tissue, and realizing the shrinkage and reduction of adipose tissue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Formulation screening, drug loading and cellular uptake of nanoemulsions
[0042] (1) Formulation screening and physicochemical properties characterization of drug-loaded nanoemulsion
[0043] Table 1: Formulation composition of nanoemulsion
[0044]
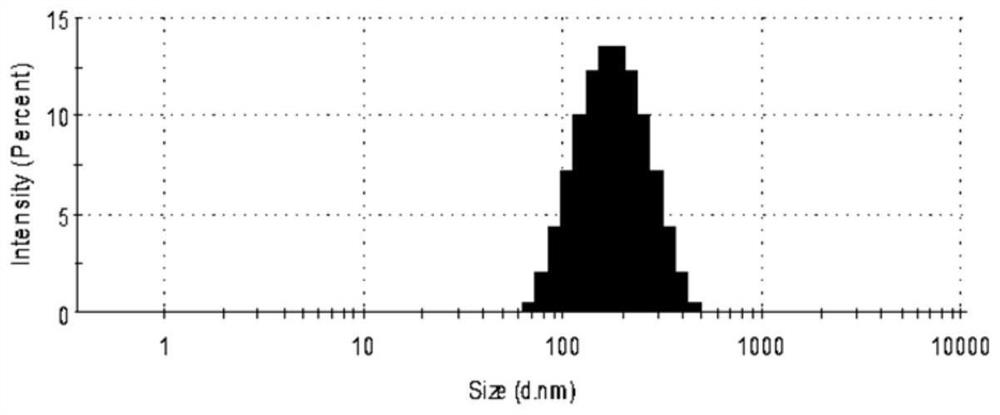
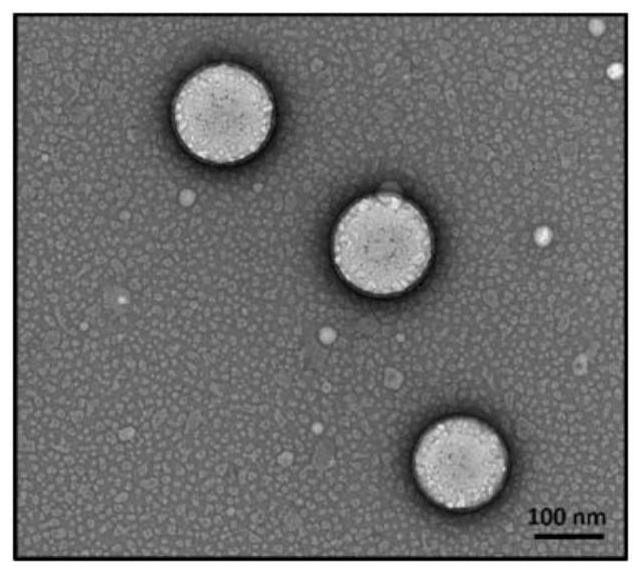
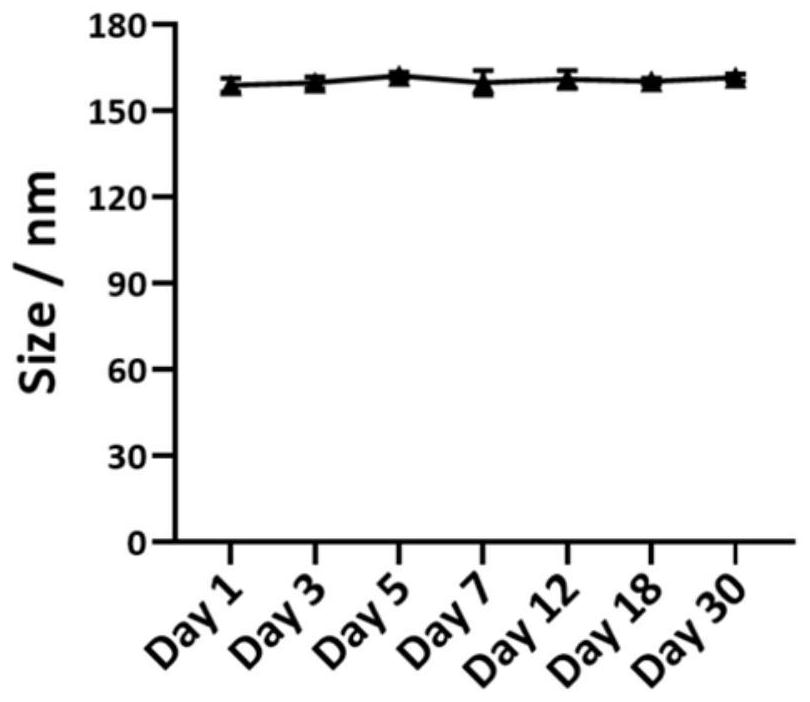
[0045] First, drug-loaded nanoemulsion (KT-NE) was prepared by emulsification ultrasonic method, and its formulation was screened. By changing the type of phosphatidylcholine (PL100M and E-80), the proportion of each lipid component and the content of the water phase, it was screened out that the stability was higher, that is, when PL100M was used as the membrane material in formulation 5 (PL100M:α- T:MCT=0.98%:0.17%:0.81%, w / w) for follow-up studies. The particle size of the nanoemulsion under this formulation is about 150 nm (detected by dynamic light scattering) (see figure 1 ); typical emulsion morphology under transmission electron microscopy (TEM) (see figure 2 ); high stability and easy storage, n...
Embodiment 2
[0050] Example 2 Validation of drug-loaded nanoemulsion inhibiting adipocyte proliferation and accumulation of lipid droplets
[0051] Taking prescription 5 of Example 1 as an example, the adipocytes or adipocytes at different differentiation stages were treated with the drug-loaded nanoemulsion KT-NE until the same batch of untreated 3T3-L1 cells had been differentiated normally into The adipocytes that can accumulate lipid droplets and have remained fully differentiated adipocytes for a week, were photographed morphologically under white light with a fluorescent inverted microscope on the 2 / 4 / 7 days after the completion of differentiation of the same batch of control adipocytes. During the period, the adipocyte family members in the experimental group treated with KT-NE maintained normal induction and differentiation measures and culture methods, and observed the inhibitory level of drug-loaded nanoemulsion in inhibiting adipocyte proliferation and lipid droplet accumulation....
Embodiment 3
[0052] Example 3 Drug-loaded nanoemulsion inhibits oxidative stress and ER stress and attenuates lipid accumulation
[0053] Nanoemulsion prescription:
[0054] Egg yolk lecithin E80 14-30mg
[0055] Cholesterol 2-10mg
[0056] Triglycerides 6mg
[0057] Alpha-Tocopherol 2mg
[0058] KIRA8 0.6-5mg
[0059] Water 1mL.
[0060] KIRA8 is an IRE1α kinase and RNase inhibitor that effectively attenuates downstream XBP1 mRNA splicing and associated lipid bulk synthesis. The adipose precursor cells or adipocytes at different differentiation stages were treated with the drug-loaded nanoemulsion KT-NE under this prescription until the same batch of untreated 3T3-L1 cells had been differentiated normally into lipid droplet-accumulating cells. adipocytes, and has accumulated a large amount of lipids in the cells. During the administration period, the adipocyte family members in the experimental group maintained normal induction and differentiation measures and culture methods. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com