Avantrombopag maleate pharmaceutical preparation as well as preparation method and application thereof
A technology for avatrombopag and pharmaceutical preparations, which is applied in the field of avatrombopag maleate and its preparation, can solve the problems of easy clogging of screens, low efficiency, low solubility and the like, and achieves difficulty in aggregation and cost savings. , The effect of quality control and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
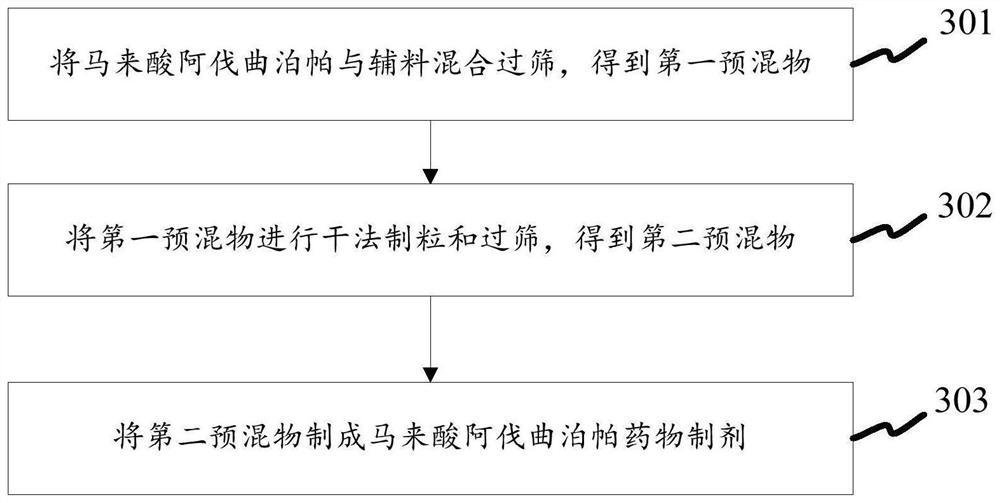
[0044] like image 3 As shown, the preparation method of the pharmaceutical preparation of avatrombopag maleate also provided by the present invention comprises:
[0045] Step 301: Mix and sieve avatrombopag maleate and auxiliary materials to obtain a first premix. The first premix can be obtained by mixing avatrombopag maleate with the auxiliary materials, and then sieving by means of rotary granulation. During the sieving process, the aperture of the screen can be a screen with a diameter of 1.2 mm to 0.8 mm. Since the average aspect ratio of avatrombopag maleate is (2-10):1, the particle size distribution is D90≤20μm, and the adhesion degree is low when D50≤10μm, it is not easy to generate electrostatic adsorption during the sieving process , thereby reducing the probability of blocking the screen holes of the screen or getting stuck in the screen holes.
[0046] Step 302: Dry granulating and sieving the first premix to obtain a second premix.
[0047] Step 303: The sec...
Embodiment 1
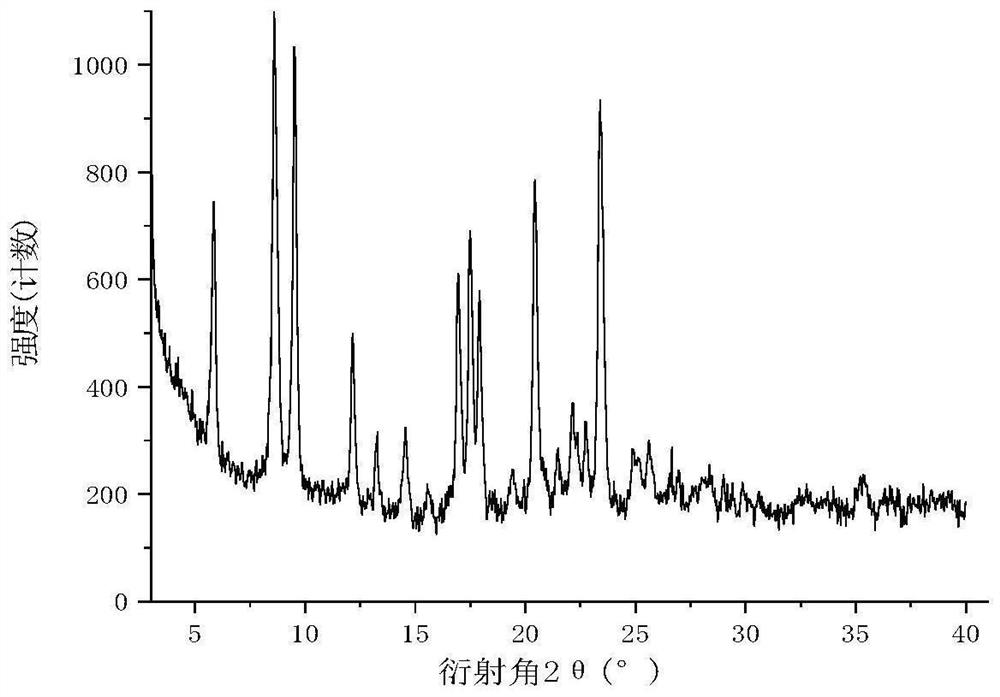
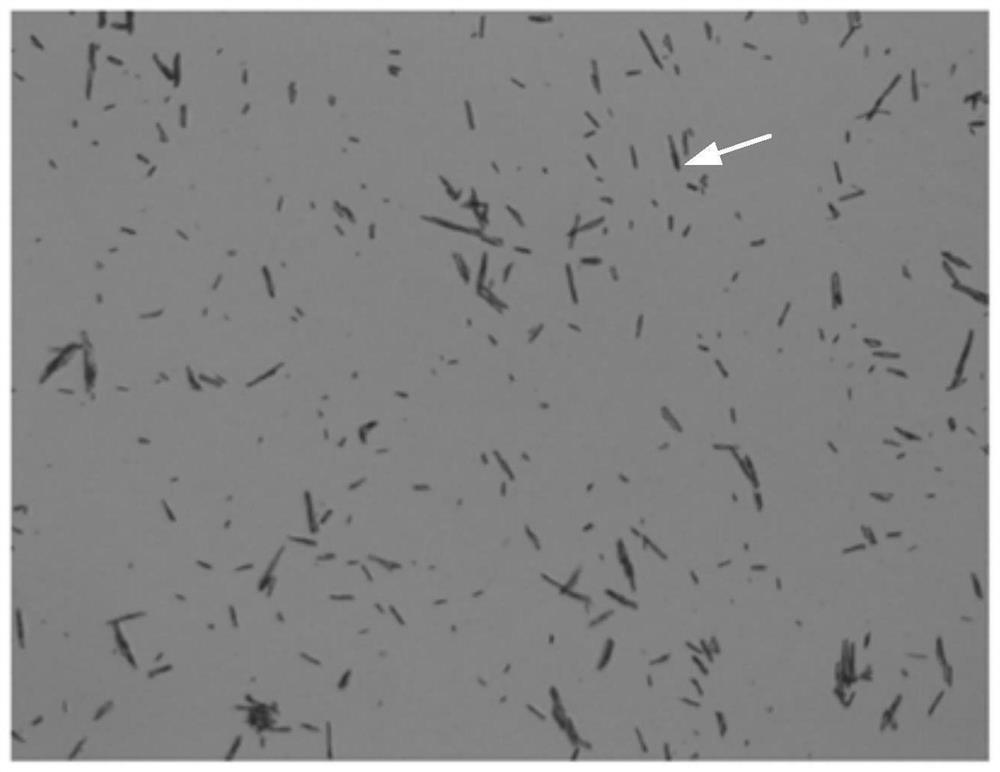
[0055] The embodiment of the present invention provides a pharmaceutical oral preparation of avatrombopag maleate, the selected active ingredient is avatrombopag maleate, the particle size distribution is D50: 10 μm, D90: 20 μm; shape or rod; the average aspect ratio is 10:1; the crystal form is C type. The specification of the pharmaceutical preparation of avatrombopag maleate in the embodiment of the present invention is 20 mg, and Table 2 shows the composition ratio table of the oral pharmaceutical preparation of avatrombopag maleate at 20 mg in the embodiment of the present invention.
[0056] Table 2 Composition ratio table of avatrombopag maleate pharmaceutical oral preparation in 20mg of Example 1
[0057]
[0058]
[0059] Due to the good oral absorption of avatrombopag maleate and the stable supply of avatrombopag maleate in mass production such as industrial production It exists in the form of popa, therefore, when recording the mass of avatropopa maleate, cho...
Embodiment 2
[0065] The particle size distribution of avatrombopag maleate is D50: 8μm, D90: 15μm; the crystal habit is needle-like or rod-like; the average aspect ratio is 8:1; the crystal form is C type. The specification of the pharmaceutical preparation of avatrombopag maleate of the embodiment of the present invention is 20 mg, and Table 3 shows the composition ratio table of the oral pharmaceutical preparation of avatrombopag maleate of the embodiment of the present invention at 20 mg.
[0066] Table 3 The composition ratio table of the oral preparation of avatrombopag maleate in 20mg of embodiment two
[0067]
[0068] The preparation method of avatrombopag maleate oral pharmaceutical preparation provided in the embodiment of the present invention comprises the following steps:
[0069] In the first step, avatrombopag maleate is mixed with excipients lactose monohydrate SuperTab 11SD, microcrystalline cellulose Vivapur101, colloidal silicon dioxide, talc and crospovidone SH-SL10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com