Preparation method of high diastereoisomer phosphonyl chloride
A technology of diastereoisomers and phosphonyl chlorides, which is applied in the field of medicinal chemistry, can solve the problems of process stability and molecular utilization that need to be further improved, low conversion rate of high diastereoisomer monophenylphosphonyl chlorides, non- Problems such as difficulty in the stability of the symmetrical conversion reaction process, etc., to achieve the effects of simple operation, improved purity, and shortened time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
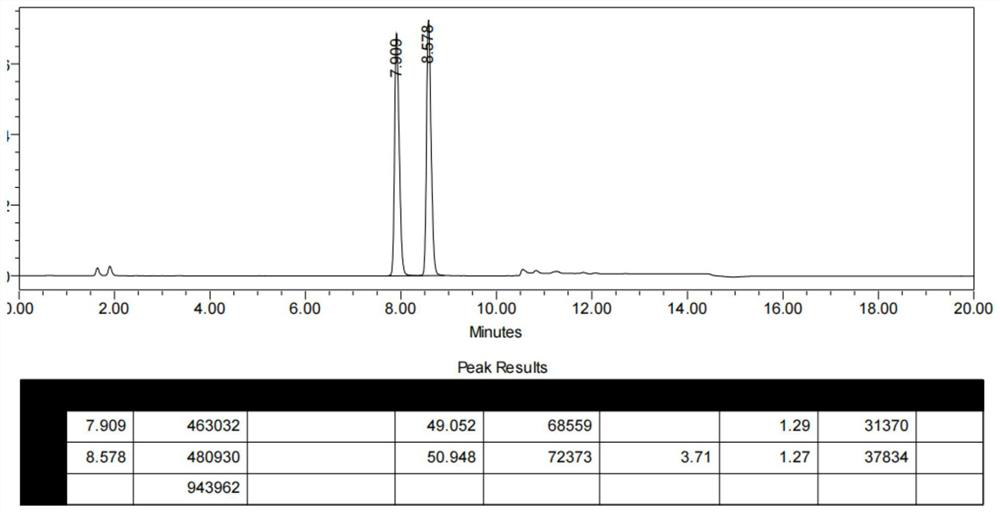
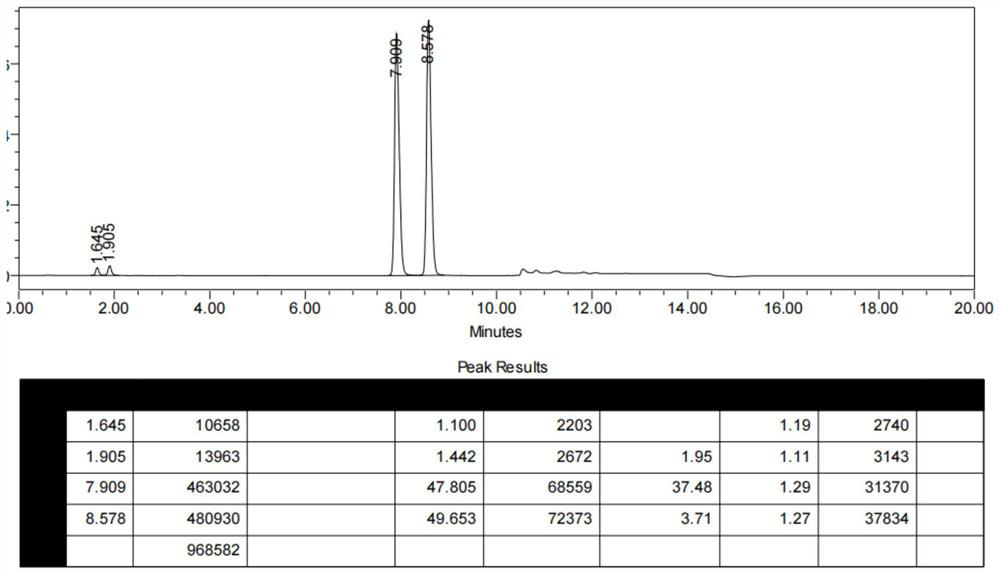
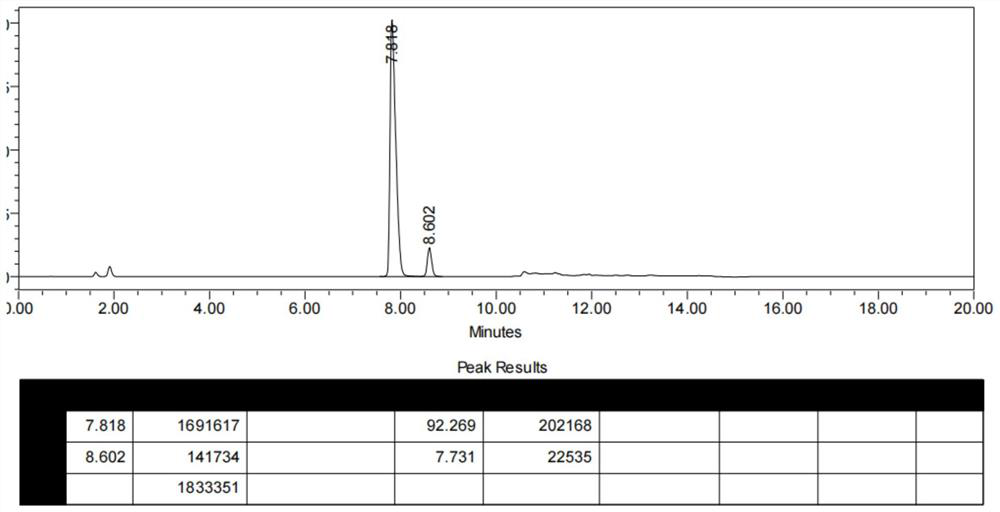
[0043] 1) At room temperature, put compound I (10 g, 0.028 mol), acetonitrile (77 mL), thionyl chloride (8.2 g, 0.069 mol) into a 250 ml reactor under nitrogen protection, heat up to 70 ° C and keep the reaction for 4 h. The racemate of compound II was obtained (HPLC analysis of the S of II P :R P =49:51); underpressure distillation, slowly concentrating to remove the reaction solvent acetonitrile, until the reaction system is slurry or solid;
[0044] 2) add anhydrous dichloromethane (50g) to the system, be warming up to about 40 DEG C and reflux stirring and beating for 1 hour to obtain a slurry with uniform particle distribution of compound II; again slowly distill and concentrate to remove dichloromethane to obtain the slurry of compound II;
[0045] 3) Add anhydrous toluene (80g, 8w) to the system, stir at 60° C. and slowly distill and concentrate again to remove toluene and residual solvent acetonitrile and dichloromethane to obtain the solid of compound II;
[0046] 4...
Embodiment 2
[0048] 1) At room temperature, put compound I (15 g, 0.041 mol), acetonitrile (115 mL), thionyl chloride (12.28 g, 0.103 mol) into a 500 ml reactor under nitrogen protection, heat up to 70 ° C and keep the reaction for 4 h. The racemate of compound II was obtained (HPLC analysis of the S of II P :R P =49:51); underpressure distillation, slowly concentrating to remove the reaction solvent acetonitrile, until the reaction system is slurry or solid;
[0049] 2) Add anhydrous dichloromethane (150g, 10w) to the system, heat up to about 40° C., reflux, stir and beat for 1 hour to obtain a slurry with uniform particle distribution of compound II, and again slowly distill and concentrate to remove dichloromethane to obtain compound II. slurry or solid;
[0050] 3) Add anhydrous toluene (120g, 8w) to the system, stir at 60°C and again slowly distill and concentrate to remove toluene and residual solvent acetonitrile and dichloromethane to obtain the slurry or solid of compound II;
...
Embodiment 3
[0053] 1) At room temperature, put compound I (20 g, 0.055 mol), acetonitrile (154 mL), thionyl chloride (16.4 g, 0.137 mol) into a 500 ml reactor under nitrogen protection, heat up to 70 ° C and keep the reaction for 4 h. The racemate of compound II was obtained (HPLC analysis of the S of II P :R P =48.5:51.5); distillation under reduced pressure, slowly concentrating to remove the reaction solvent acetonitrile, until the reaction system is slurry or solid;
[0054] 2) add anhydrous toluene (160g, 8w) to the system, be warming up to about 60 DEG C and keep stirring and beating for 1.5 hours to obtain a slurry with uniform particle distribution of compound II, and again slowly distill and concentrate to remove toluene to obtain the slurry of compound II;
[0055] 3) adding anhydrous toluene (160g, 8w) to the system, stirring at 60°C and slowly distilling and concentrating again to remove toluene and residual solvent acetonitrile to obtain the slurry or solid of compound II; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com