Cs < + > and Sr < 2 + > co-adsorption-separation difunctional ion exchanger as well as preparation method and application thereof
An ion exchanger and co-adsorption technology, applied in cation exchange materials, ion-exchange water/sewage treatment, chemical instruments and methods, etc. Poor alkali tolerance and other problems, to achieve the effect of fast adsorption rate, sensitive pH sensitivity, strong acid-base tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Preparation of K-SbS ion exchanger and product characteristics
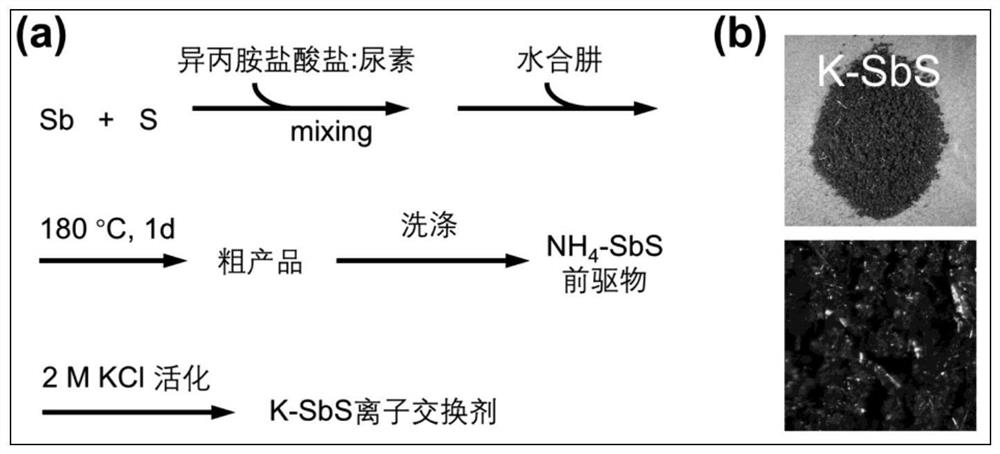
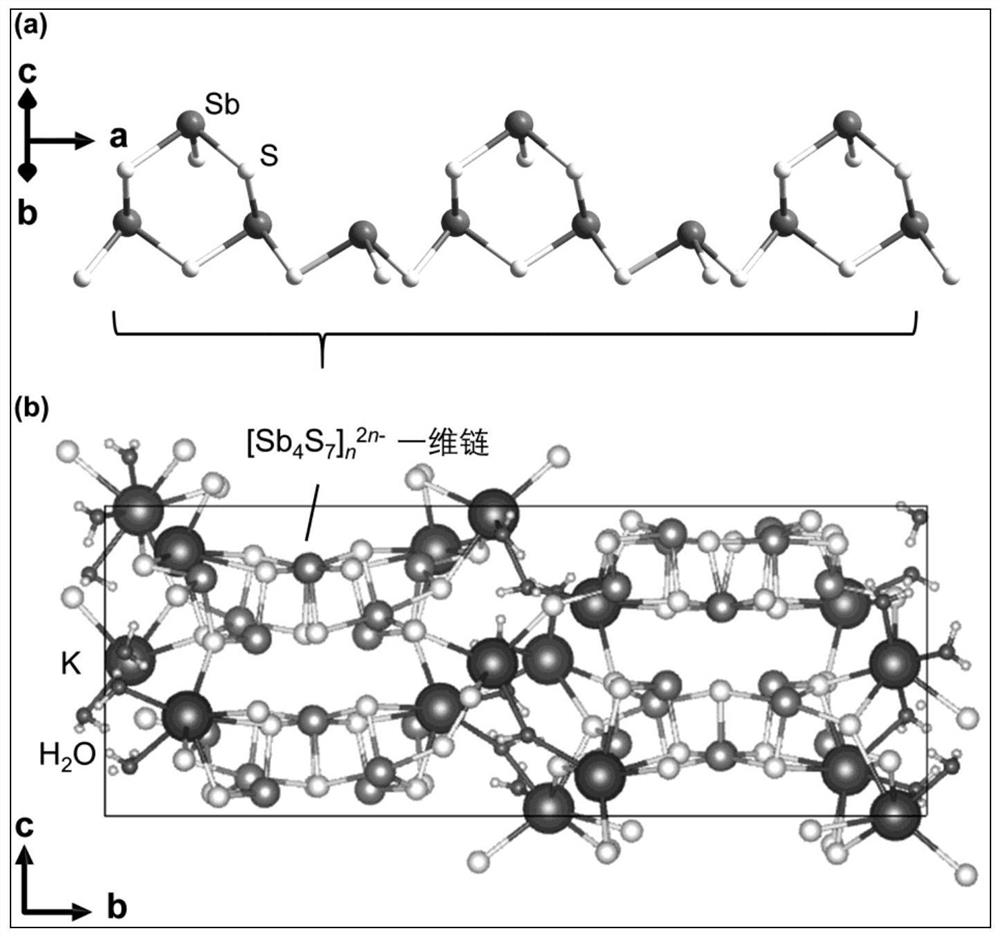
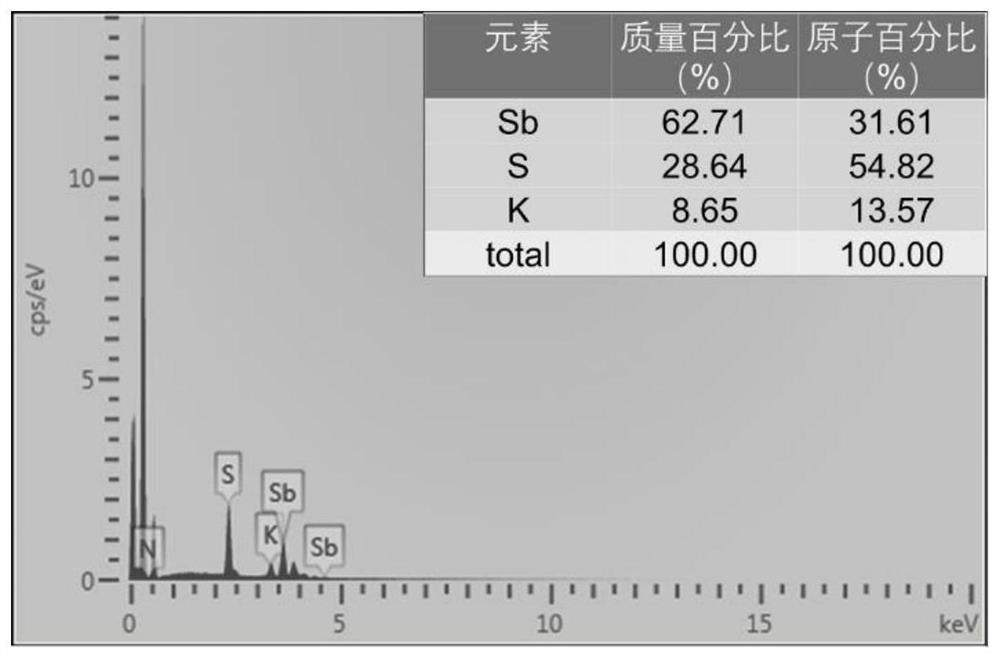
[0075] like figure 1 As shown, 1.0 mmol of antimony powder, 4.0 mmol of sulfur powder, 8.0 mmol of isopropylamine hydrochloride, 16.0 mmol of urea and 12 mmol (about 0.6 mL) of hydrazine hydrate (98%) were thoroughly mixed, and then transferred to In a reaction kettle equipped with 20 mL of polytetrafluoroethylene lining, the reaction was heated at 180 ° C for 24 h. After the reaction was completed, it was naturally cooled to room temperature. NH 4 -SbS precursor; take 0.5g NH 4 -SbS precursor was added to 20mL potassium chloride solution (2M), after stirring at room temperature for 24h, the solid product was taken out by filtration, washed with 20mL water for 2 times, washed with 20mL ethanol for 2 times, and then naturally dried to obtain K-SbS ion exchanger , the calculated yield based on Sb was 86.5%.
[0076] Wherein, yield=(mass of obtained K-SbS ion exchanger sample / product molecular w...
Embodiment 2
[0085] Example 2K-SbS ion exchanger on single Cs + , Sr 2+ adsorption kinetics of ions
[0086] Take samples K-SbS and 6ppm containing single Cs + , Sr 2+ The aqueous solution of ions was mixed and stirred continuously for 8h at room temperature (20°C), V / m=1000mL g -1 , pH 6-7. A small amount of the mixture supernatant was taken at regular intervals, and after dilution, Cs was determined by ICP-MS. + , Sr 2+ ion concentration. Sampling NH under the same conditions 4 -SbS was tested as a reference. like Image 6 (a), Image 6 As shown in (b), the precursor NH 4 -SbS for Cs + , Sr 2+ The adsorption rate of ions is slow and the removal rate is low. At 480min, Cs + , Sr 2+ The removal rates were 79.4% and 11.5%, respectively. However, the adsorption speed of the activated K-SbS ion exchanger was significantly improved, and more than 93% of Cs could be removed within 2 min and 20 min, respectively. + and Sr 2+ ions, 40min to reach adsorption equilibrium, Cs at e...
Embodiment 3
[0087] Example 3K-SbS ion exchanger on single Cs + , Sr 2+ Ion adsorption isotherm
[0088] Take sample K-SbS with 2-769ppm of single Cs + or 3-776ppm of single Sr 2+ mixed with the aqueous solution, and continued stirring at room temperature (20 °C) for 8 h, V / m = 1000 mL g -1 , pH 6-7. The initial solution and the reacted supernatant were taken respectively, and the Cs were determined by ICP-MS after dilution. + , Sr 2+ ion concentration. Sampling NH under the same conditions 4 -SbS was tested as a reference. The experimental results are as Figure 7 (a), Figure 7 As shown in (b), the adsorption isotherm curve was fitted by the Langmuir-Frendlich model, NH 4 -SbS vs Cs + and Sr 2+ The saturated adsorption capacities of the ions were 70.96 and 49.48 mg g, respectively -1 , while the activated K-SbS ion exchanger has a great effect on Cs + and Sr 2+ The saturated adsorption capacity of ions has increased significantly, reaching 318.77 and 61.12 mg g, respectiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Saturated adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com