Recombinant pichia pastoris for producing human hyaluronidase PH20 and construction method of recombinant pichia pastoris
A technology of hyaluronidase and Pichia pastoris, which is applied in the field of genetic engineering, can solve problems such as inability to meet application requirements, and achieve the effects of reducing intraocular pressure, promoting diffusion and absorption, and increasing permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Construction of hPH20 and its mutants
[0048] According to the hPH20 sequence reported by NCBI (Genbank accession number: NP_003108.2), the nucleotide sequence corresponding to the 36-511th amino acid sequence was optimized according to the Pichia pastoris codon preference. The optimized gene sequence SEQ ID NO .1 It was synthesized by Universal Biosystems (Anhui) Co., Ltd. and ligated between the EcoRI and Not I restriction sites of the expression vector pPIC9K to obtain the recombinant plasmid pPIC9K-hPH20. Next, the truncation mutation primers △484C-F, △484C-R and △491C-F, △491C-R as shown in Table 1 were designed, using the plasmid pPIC9K-hPH20 as the template, using PCR technology, respectively construct the C deletion hPH20 Truncated mutants of amino acids after position 484 (that is, retaining position 483, truncating positions 484 and 484) and amino acids after position 491 (that is, retaining position 490, truncating position 491 and later) △484C an...
Embodiment 2
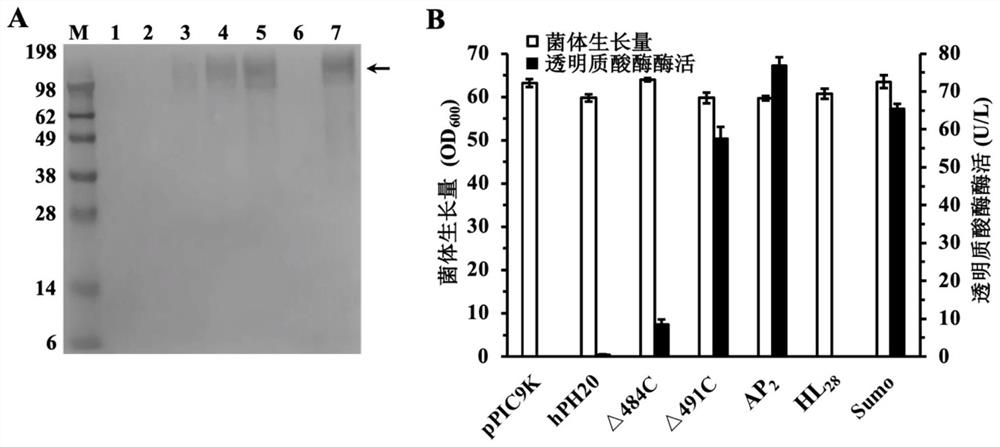
[0065] Example 2 Shake flask fermentation of hPH20 and its mutants
[0066] The recombinants P. pastoris-pPIC9K-hPH20, P. pastoris-pPIC9K-Δ484C, P. pastoris-pPIC9K-Δ491C, P. pastoris-pPIC9K-ap constructed in Example 1 2 -△491C, P.pastoris-pPIC9K-hl 28 -△491C and P. pastoris-pPIC9K-sumo-△491C were streaked on the YPD solid plate and placed in a 30°C constant temperature incubator until a single colony was grown. A single colony was inoculated into a 250 mL shake flask containing 50 mL of YPD liquid medium, and cultured overnight at 30° C. and 200 rpm to obtain a seed culture. The seed culture was transferred to a 250 mL shake flask containing 50 mL of BMGY medium at 10% (v / v) inoculum, and cultivated to OD at 30 °C and 200 rpm 600 When reaching 6, collect all the cells and wash them with 0.9% NaCl for 3 times, then transfer them to a 250 mL shake flask containing 50 mL of BMMY medium, and cultivate at 30 °C and 200 rpm for 96 h, supplemented with a final concentration of 1% (...
Embodiment 3
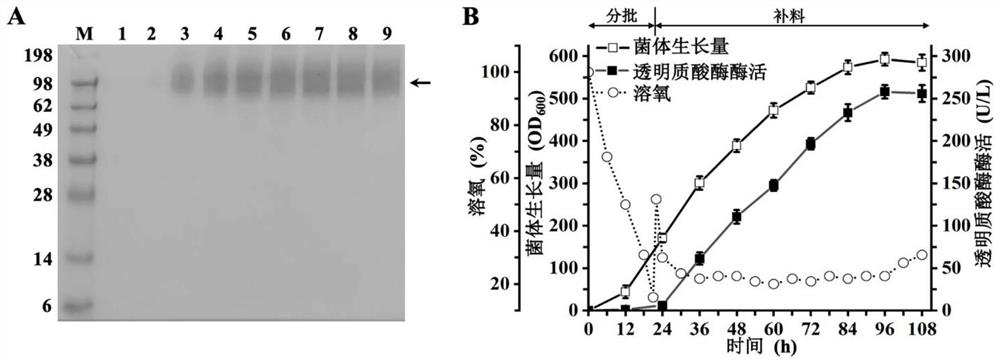
[0073] Example 3 Mutant AP 2 - △ 491C 3-L fermenter test
[0074] The recombinant P. pastoris-pPIC9K-ap constructed in Example 1 2 -△491C was streaked on the YPD solid plate and placed in a 30°C constant temperature incubator until a single colony was grown. A single colony was inoculated into a 250 mL Erlenmeyer flask containing 50 mL of YPD liquid medium, and cultured overnight at 30°C and 200 rpm. The seed culture was transferred to a 3-L fermentor containing 900 mL of BSM medium at a 10% (v / v) inoculum with initial parameter settings: temperature 30°C, pH 5.0, aeration 2.0 vvm and rotation speed 200 rpm. When the glycerol was exhausted and the dissolved oxygen in the fermentation broth rebounded, the glycerol mother liquor with a concentration of 500g / L containing 1.2% PTM1 by volume was added at a rate of 25mL / L / h, and the feed was fed for 10h. During the process, the ventilation volume was adjusted as 4.0vvm, the rotation speed was gradually increased to 800-900rpm ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com