Freeze-drying preparation as well as preparation method and application thereof
A technology of freeze-dried preparations and freeze-dried excipients, applied in the field of medicine, can solve the problems of poor storage stability of finished products, inability to ensure complete reconstitution, long reconstitution time, etc., and achieve good storage stability, short reconstitution time, The effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
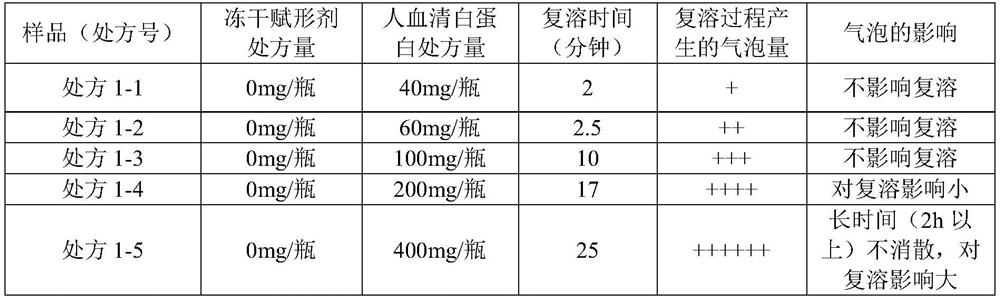
[0231] 1.1 Prescribing Information
[0232] Raw materials Prescription 1-1 Prescription 1-2 Prescription 1-3 Prescription 1-4 Prescription 1-5 Fosaprepitant dimeglumine 1.545g 1.545g 1.545g 1.545g 1.545g human serum albumin 240mg 360mg 600mg 1200mg 2400mg Freeze-dried excipients 0mg 0mg 0mg 0mg 0mg 0.5mol / L sodium hydroxide Moderate Moderate Moderate Moderate Moderate purified water to 36mL to 36mL to 36mL to 36mL to 36mL
[0233] 1.2 Preparation process
[0234] In a 100mL beaker (including a rotor), add an appropriate amount of water (10°C to 30°C) and the prescribed amount of human serum albumin, and start stirring. The total solution of this step is 88.33% of the prepared amount.
[0235] Weigh the prescribed amount of fosaprepitant dimeglumine and add it to the above 100mL beaker to dissolve it.
[0236] Slowly add 0.5mol / L sodium hydroxide solution dropwise to adjust the pH of ...
Embodiment 2
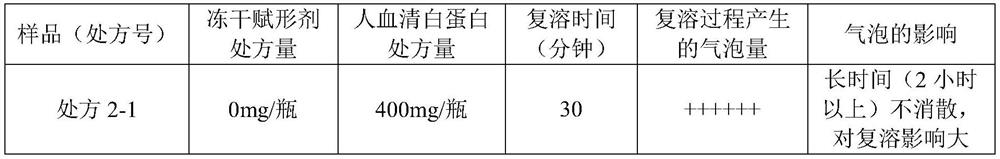
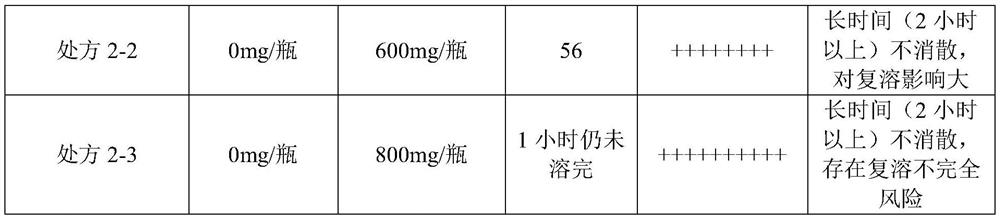
[0247] 2.1 Prescribing Information
[0248] Raw materials Prescription 2-1 Prescription 2-2 Prescription 2-3 Fosaprepitant dimeglumine 1.0303g 1.0303g 1.0303g human serum albumin 1600mg 2400mg 3200mg Freeze-dried excipients 0mg 0mg 0mg 0.5mol / L sodium hydroxide Moderate Moderate Moderate purified water to 24mL to 24mL to 24mL
[0249] 2.2 Preparation process
[0250] In a 50mL beaker (including a rotor), add an appropriate amount of water (10°C to 30°C) and the prescribed amount of human serum albumin, and start stirring. The total solution of this step is 88.33% of the prepared amount.
[0251] Weigh the prescribed amount of fosaprepitant dimeglumine and add it to the above-mentioned 50 mL beaker to dissolve it.
[0252] Slowly add 0.5mol / L sodium hydroxide solution dropwise to adjust the pH of the medicinal solution to 8.50-9.00.
[0253] Add water to make up the total amount, stir evenly, filter the l...
Embodiment 3
[0264] 3.1 Prescribing Information
[0265] Raw materials Prescription 3-1 Prescription 3-2 Prescription 3-3 Prescription 3-4 Prescription 3-5 Fosaprepitant dimeglumine 1.545g 1.545g 1.545g 1.545g 1.545g Palonosetron Hydrochloride 1.764mg 1.764mg 1.764mg 1.764mg 1.764mg human serum albumin 360mg 480mg 600mg 1200mg 2400mg Freeze-dried excipients 0mg 0mg 0mg 0mg 0mg 0.5mol / L sodium hydroxide Moderate Moderate Moderate Moderate Moderate purified water to 36mL to 36mL to 36mL to 36mL to 36mL
[0266] 3.2 Preparation process
[0267] In a 100mL beaker (including a rotor), add an appropriate amount of water (10°C to 30°C), the prescribed amount of human serum albumin, and the prescribed amount of palonosetron hydrochloride, and start stirring. The total solution in this step is 88.33% of the preparation amount.
[0268] Weigh the prescribed amount of fosaprepitant dimeglumine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com