Molecular switch with multiple stimulation responses and synthesis method thereof
A technology of multiple stimuli responses and synthesis methods, applied in chemical instruments and methods, analyzing materials through chemical reactions, and analyzing materials through observing the influence of chemical indicators, can solve the problems of poor reversibility and poor reaction yields. High-level problems, to achieve the effect of rapid reaction, simple synthesis route, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
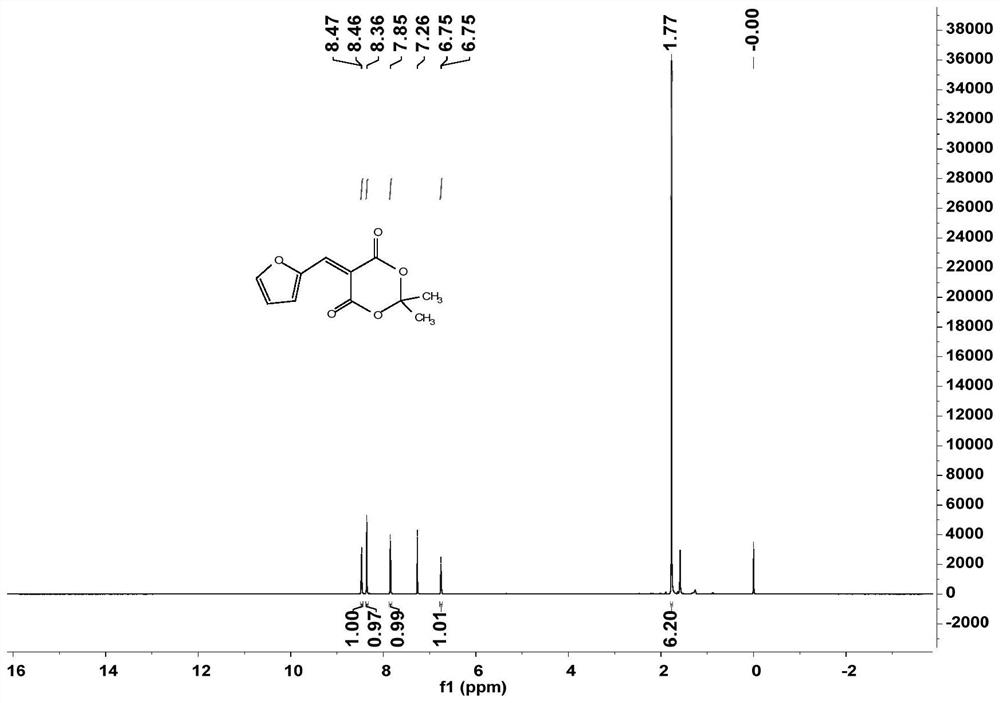
[0035] (1) Compound I of the present invention is 5-(furan-2-ylmethylene)-2,2-dimethyl-1,3-dioxane-4,6-dione, and its molecular structure is as follows Show:
[0036]
[0037] Prepared by the following steps:
[0038] In the double-necked flask, according to the molar ratio of 2-furancarboxaldehyde: cycloisopropylmalonate=1.1:1, firstly add cycloisopropylidene malonate into the flask, then add deionized water as a solvent, 2-furancarboxaldehyde was slowly added dropwise with stirring, and the reaction was carried out at 25° C. for 2 h. After the reaction, add CH 2 Cl 2 Extraction of crude product, CH 2 Cl 2 The aqueous phase was extracted (25ml*3), and the organic phase was collected. Saturated NaCl solution and saturated NaHSO were added successively. 4 , saturated NaHCO 3 solution, for CH 2 Cl 2 The phase was washed, and then a small amount of water in the organic phase was dried with anhydrous sodium sulfate, the solvent was removed under reduced pressure, and ...
Embodiment 2
[0044] Reversible photoresponse test of compound II:
[0045] Under visible light irradiation (>535nm), in toluene solution, the photoisomerization of compound II (in toluene, c=1.0×10 - 5 mol / L). Its performance characteristics see Figure 7-8 .
[0046] Figure 7 a is the absorption spectrum of compound II in different concentrations of toluene as a function of concentration, and the maximum absorbance is at 544 nm. b is a linear relationship between the absorbance at 544nm and the concentration of compound II. This shows that the toluene solution of compound II has a good linear relationship and the maximum absorption wavelength does not change with the concentration.
[0047] from Figure 8 It can be seen that the absorbance of compound II decreased to 0.1 under the illumination of >535nm light for 1min, and then placed in the dark, the absorbance would rise slowly, and the absorbance reached the maximum after being placed for 20min. Then repeat the light until the a...
Embodiment 3
[0049] Reversible acid-base response test of compound II:
[0050] Figure 9a It is the color change diagram of the acid-base response of compound II in DMSO solution, 1 is the initial state of the sample; 2 is the state after adding equimolar amount of base; 3 is the state after adding equimolar amount of acid. b: Compound Ⅱ in DMSO-d 6 The NMR test of adding acid and adding base is carried out in the figure, and the characteristic NMR spectrum of the conjugated olefin region of compound II is intercepted in the figure, 1 is the initial state of the sample; 2 is the state after adding an equimolar amount of base; 3 is an equimolar amount added state after acid. This shows that compound Ⅱ can obtain an intermediate isomer by adding alkali in DMSO, and then can be restored to the original state by adding acid, showing a good switching potential, and can carry out many cycles, and has excellent anti-fatigue performance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com