Synthesis method of dihydrooxazole compound
A technology of dihydrooxazole and synthetic methods, which is applied in chemical instruments and methods, chemical/physical processes, chemical/physical/physical chemical processes, etc., can solve the problems of cumbersome reaction steps, low reaction efficiency, long reaction time, etc. To achieve the effect of shortening the reaction time, speeding up the reaction rate and green reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
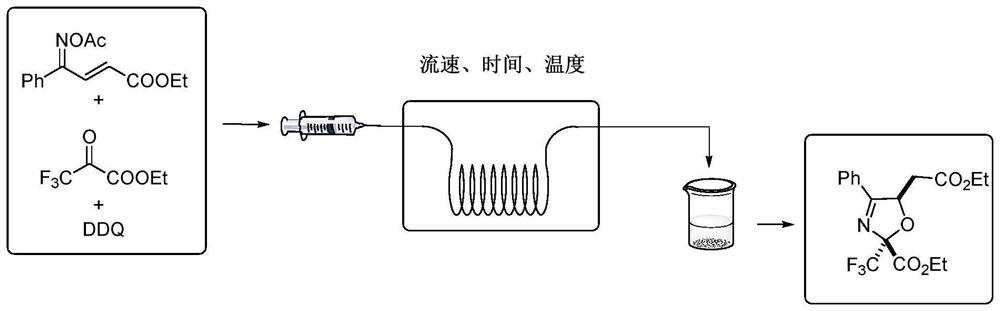
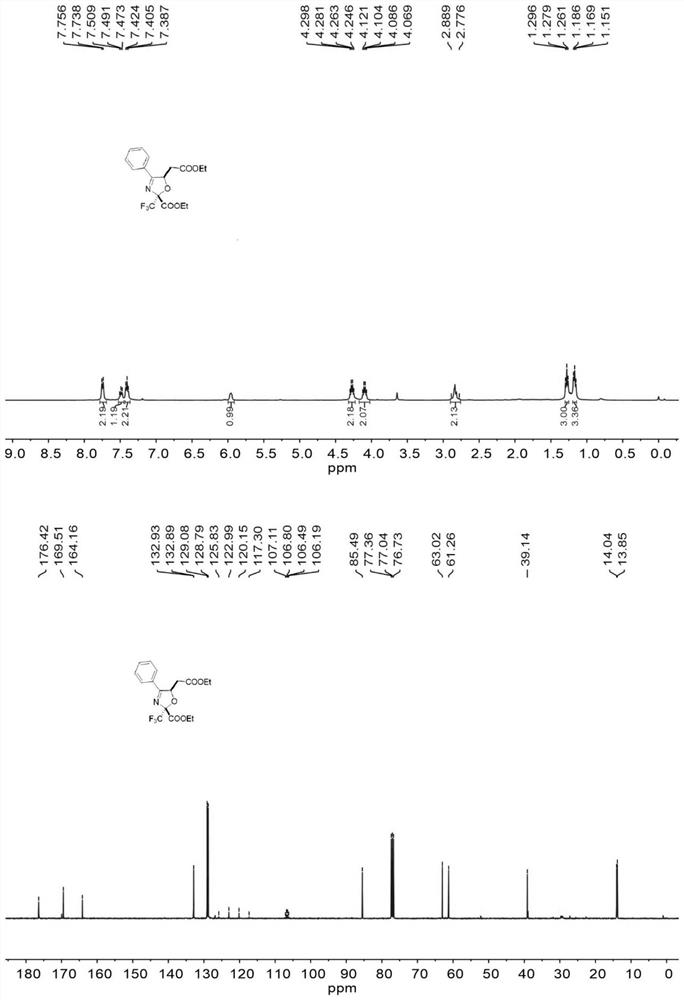
[0039] 1 mmol (0.261 g) of (2E,4E)-4-(acetoxyimino)-4-phenylbut-2-enoic acid ethyl ester, 4 mmol of ethyl trifluoropyruvate (0.680 g), dichloro Dicyanobenzoquinone (0.3405g) was dissolved in 20ml of toluene, and the flow rate was 0.2mL / min into the microchannel reactor (the inner diameter of the copper tube of the microreactor was 1mm and the volume of the copper tube was 4mL) and reacted at 120°C for 8min. The organic phase was obtained from the discharge of the microreactor, and the crude product was obtained by vacuum concentration, and separated by column chromatography with a developing solvent with a ratio of petroleum ether to ethyl acetate of 5:1, and the target product was obtained as shown in Table 1, and the yield was 87%. . 1H NMR (400MHz, Chloroform-d) δ 7.75 (d, J=7.4Hz, 2H), 7.51–7.47 (m, 1H), 7.41 (t, J=7.3Hz, 2H), 5.96 (dd, J= 7.6, 3.8Hz, 1H), 4.27 (q, J=7.0Hz, 2H), 4.10 (q, J=7.0Hz, 2H), 2.89–2.78 (m, 2H), 1.28 (t, J=7.0Hz, 3H),1.19–1.15(m,3H)ppm; 13 C NMR...
Embodiment 2
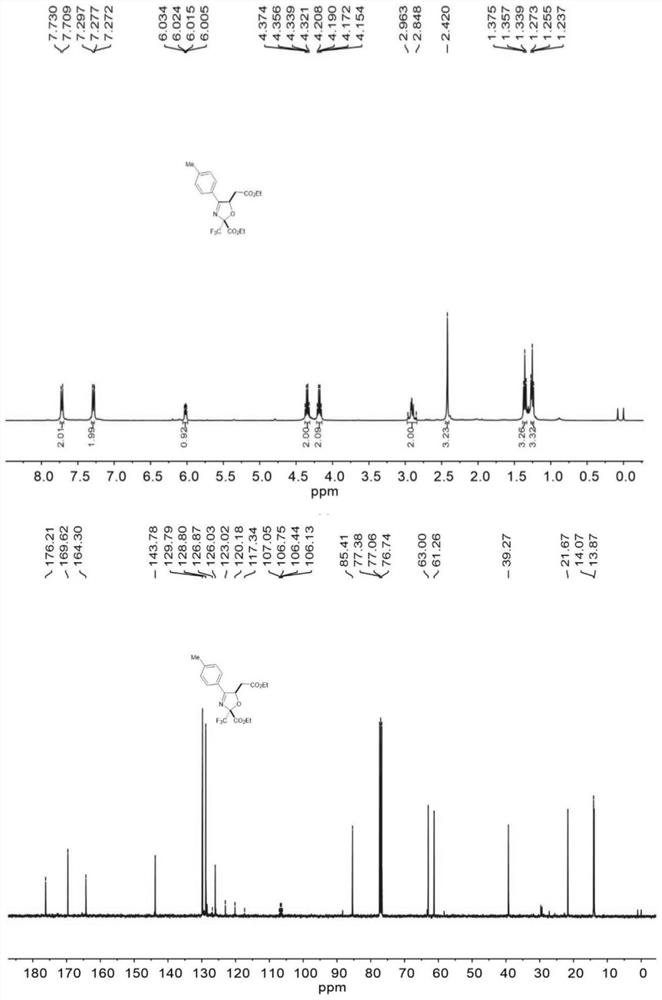
[0041] 1 mmol (0.275 g) of (2E,4E)-4-(acetoxyimino)-4-(p-tolyl)but-2-enoic acid ethyl ester, 4 mmol of ethyl trifluoropyruvate (0.680 g) , Dichlorodicyanobenzoquinone (0.3405g) was dissolved in 20ml of toluene, and the flow rate was 0.2mL / min into the microchannel reactor (the inner diameter of the copper tube of the microreactor was 1mm, and the volume of the copper tube was 4mL) at 120 ° C. The reaction was carried out for 8 minutes. The microreactor is discharged to obtain an organic phase, which is concentrated in vacuo to obtain a crude product, which is separated by a developing solvent column chromatography with a petroleum ether and ethyl acetate ratio of 5:1, and the target product can be obtained. See Table 1, and the yield is 83%. . 1 H NMR (400MHz, Chloroform-d) δ 7.72 (d, J=8.2Hz, 2H), 7.29 (d, J=8.1Hz, 2H), 6.02 (dd, J=7.6, 3.7Hz, 1H), 4.35 (q, J=7.1Hz, 2H), 4.18 (q, J=7.2Hz, 2H), 1.25 (t, J=7.1Hz, 3H), 2.96–2.85 (m, 2H), 2.42 (s, 3H) ,1.36(t,J=7.1Hz,3H)ppm; ...
Embodiment 3
[0043] 1 mmol (0.295 g) of (2E,4E)-4-(acetoxyimino)-4-(p-chlorophenyl)but-2-enoic acid ethyl ester, 4 mmol of ethyl trifluoropyruvate (0.680 g) ), dichlorodicyanobenzoquinone (0.3405g) was dissolved in 20ml of toluene, and the flow rate was 0.2 mL / min into the microchannel reactor (the inner diameter of the microreactor copper tube was 1mm, and the volume of the copper tube was 4mL) at 120°C The reaction was continued for 8 min. The microreactor is discharged to obtain an organic phase, which is concentrated in vacuo to obtain a crude product, which is separated by a developing solvent column chromatography with a petroleum ether and ethyl acetate ratio of 5:1, and the target product can be obtained. See Table 1, and the yield is 85%. . 1 H NMR (400 MHz, Chloroform-d) δ 7.78 (d, J=8.6Hz, 2H), 7.47 (d, J=8.6Hz, 2H), 6.00 (dd, J=6.4, 4.6Hz, 1H), 4.36(q,J=7.1Hz,2H),4.17(q,J=7.1Hz,2H),2.94–2.85(m,2H),1.36(t,J=7.1Hz,3H),1.25(t,J =7.1Hz, 3H)ppm; 13 C NMR (100MHz, Chloroform-d) δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com