3-chloro methyl benzoic acid synthetic method
A technology of chloromethylbenzoic acid and a synthesis method, applied in the field of preparation of organic intermediates, can solve the problems of high safety risk, complicated purification process, low yield and purity of finished products, etc., and achieves high safety, high purity, and synthetic short route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
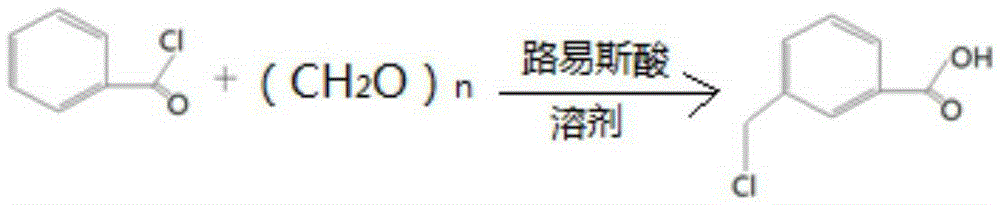
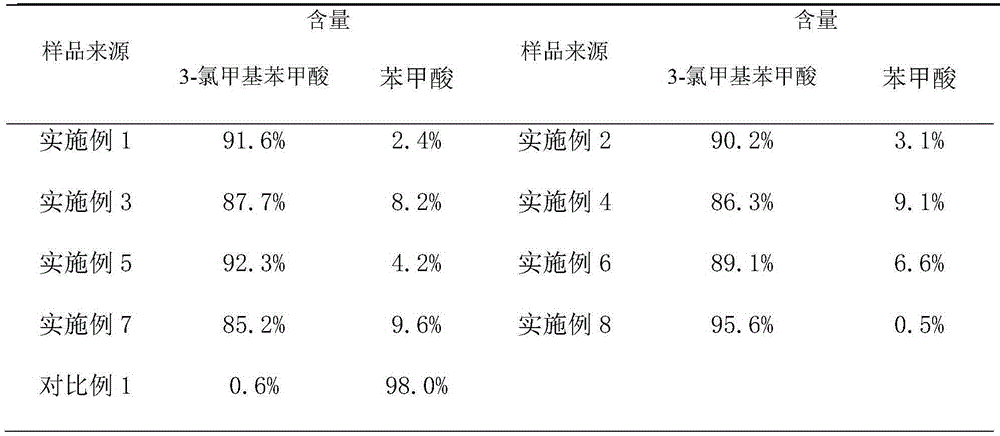
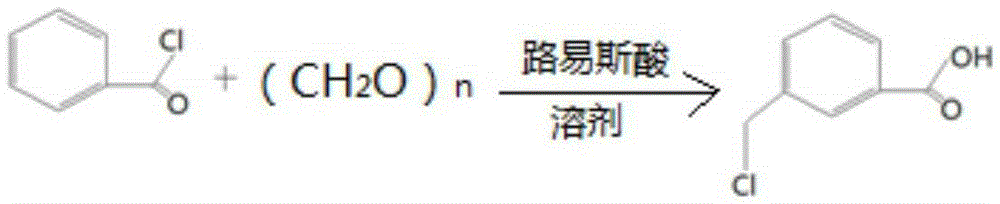
[0025] Put 100ml of dichloromethane into the autoclave, add 0.1mol of benzoyl chloride, 0.15mol of paraformaldehyde, 0.05mol of anhydrous zinc chloride, fill with nitrogen to 0.3Mpa, react at 60℃~70℃ for 15h, then cool down to At room temperature, the nitrogen gas was vented, the reaction solution was dropped into 100ml of ice water, stirred for 0.5h, and the layers were statically separated, and the lower organic layer was removed for HPLC (High Performance Liquid Chromatography, high performance liquid chromatography) analysis, the crude product contained benzoic acid 2.4% (percentage by weight) , 91.6% (weight percent) of 3-chloromethylbenzoic acid.
Embodiment 2
[0027] Put 60ml of chloroform into the autoclave, add 0.1mol of benzoyl chloride, 0.10mol of paraformaldehyde, 0.005mol of anhydrous ferric chloride, fill with nitrogen to 0.5Mpa, react at 20℃~25℃ for 10h, then cool down to room temperature , vent the nitrogen, put the reaction solution into 100ml ice water, stir for 0.5h, static layering, take the lower organic layer for HPLC analysis, the crude product contains 3.1% benzoic acid, 90.2% 3-chloromethylbenzoic acid.
Embodiment 3
[0029] Put 50ml of carbon tetrachloride into the autoclave, add 0.1mol of benzoyl chloride, 0.12mol of paraformaldehyde, 0.02mol of anhydrous aluminum trichloride, fill nitrogen to 0.1Mpa, and react at 55°C to 60°C for 20h, then Cool down to room temperature, vent the nitrogen, put the reaction solution into 100ml of ice water, stir for 0.5h, stand still and separate the layers, take the lower organic layer for HPLC analysis, the crude product contains 8.2% benzoic acid and 87.7% 3-chloromethylbenzoic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com