Fusion protein based on single-chain antibody fragment, nano assembly and preparation method and application thereof
A nano-assembly, fusion protein technology, applied in the field of medicine, can solve the problems of destroying the antigen recognition region of the antibody, reducing the antigen recognition of the antibody, complicated reaction and purification process, etc., and achieving the effect of excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] In some embodiments of the present invention, it relates to a method for preparing the above-mentioned nano-assembly, comprising the following steps:
[0068] (1) mixing the fusion protein with water or an aqueous solution to obtain an aqueous phase; mixing the hydrophobic degradable polyester and its derivatives with an organic solvent to obtain an oil phase;
[0069] (2) preparing the water phase and the oil phase described in step (1) into an oil-in-water emulsion;
[0070] (3) Separating and purifying the emulsion to obtain a nano assembly.
[0071] In some of these embodiments, an oil-in-water emulsion is prepared by means of microfluidics, high-pressure homogenization, and ultrasound;
[0072] Alternatively, the emulsion is separated and purified by means of rotary evaporation, freeze-drying, centrifugation, chromatography, ultrafiltration, etc. to obtain a nano assembly.
[0073] In some of these embodiments, the concentration of the fusion protein in the aqueo...
Embodiment 1
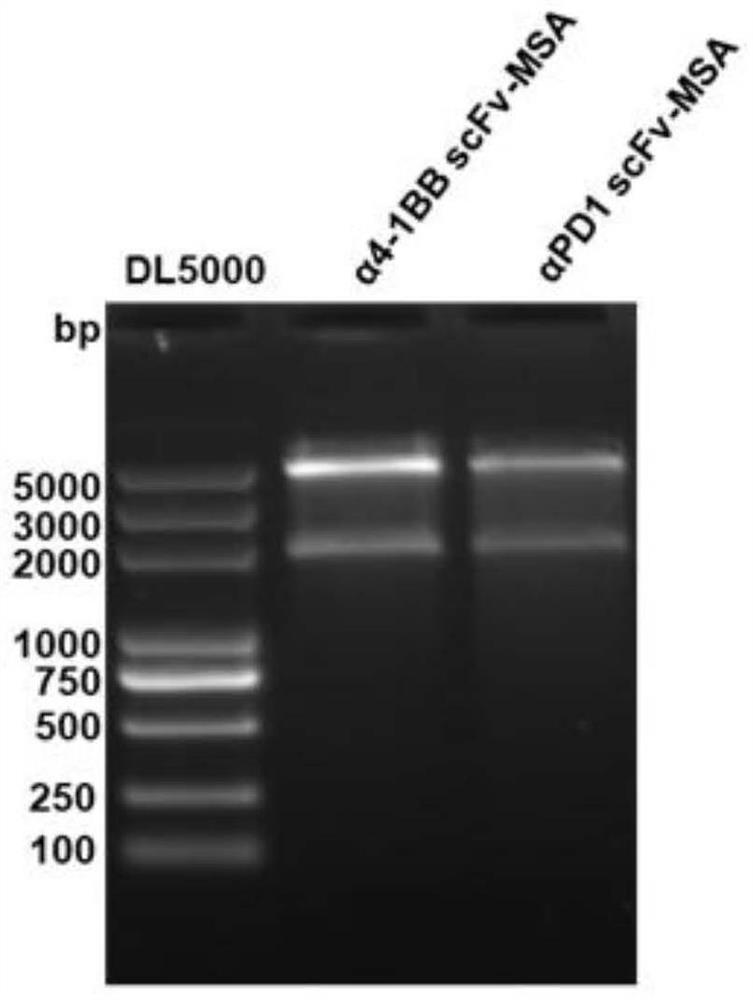
[0139] Embodiment 1 Design of single-chain antibody fragment fusion protein gene
[0140] Enter the IMGT database, enter the antibody sequence search entry, enter the antibody target to be queried, select the antibody, enter the amino acid sequence interface, select the VH and VL Kappa or Lamda segment, and use the two segments (GGGGS) 3 Linked to obtain the corresponding single-chain antibody fragment peptide sequence.
[0141] The structures of αHER2 scFv and α4-1BB scFv are VL-L-VH, and the structures of αPDL1 scFv and αTIGIT scFv are VH-L-VL.
[0142] Pass the single-chain antibody fragment with the peptide sequence of HSA or MSA without signal peptide (GGGGS) 3 The corresponding single-chain antibody fragment-albumin recombinant fusion protein peptide sequence is obtained.
[0143] The peptide sequence is converted into a nucleotide sequence by SnapGene or other similar software, which is synthesized by Shanghai Sangon.
[0144] αHER2 scFv-HSA was constructed by the abov...
Embodiment 2
[0149] Embodiment 2 Amplifies MSA sequence from mouse cDNA library
[0150] Obtain the MSA (mouse serum albumin, Mouse Serum Albumin) cDNA that does not have signal peptide coding sequence from mouse liver fetal cDNA library by PCR method, used primer MSA F (SEQ ID NO.8) and MSA R (SEQ ID NO.9) was synthesized with an oligonucleotide synthesizer, the downstream primer was introduced into the XbaI restriction site and the protective base, and the underlined part was the endonuclease recognition sequence.
[0151] 50μL PCR reaction system: 2x Mix 25μL, DNA template 2 O is supplemented, and the reaction system can be reduced or enlarged according to the demand. Perform PCR after gentle mixing. The PCR reaction conditions are heat denaturation at 94°C for 1 min; denaturation at 94°C for 30 s; annealing at 58°C for 30 s; extension at 72°C for 1.5 min; a total of 30 cycles; and extension at 72°C for 5 min. The expected 1.6kb band was obtained by 1% agarose gel detection analysis, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com