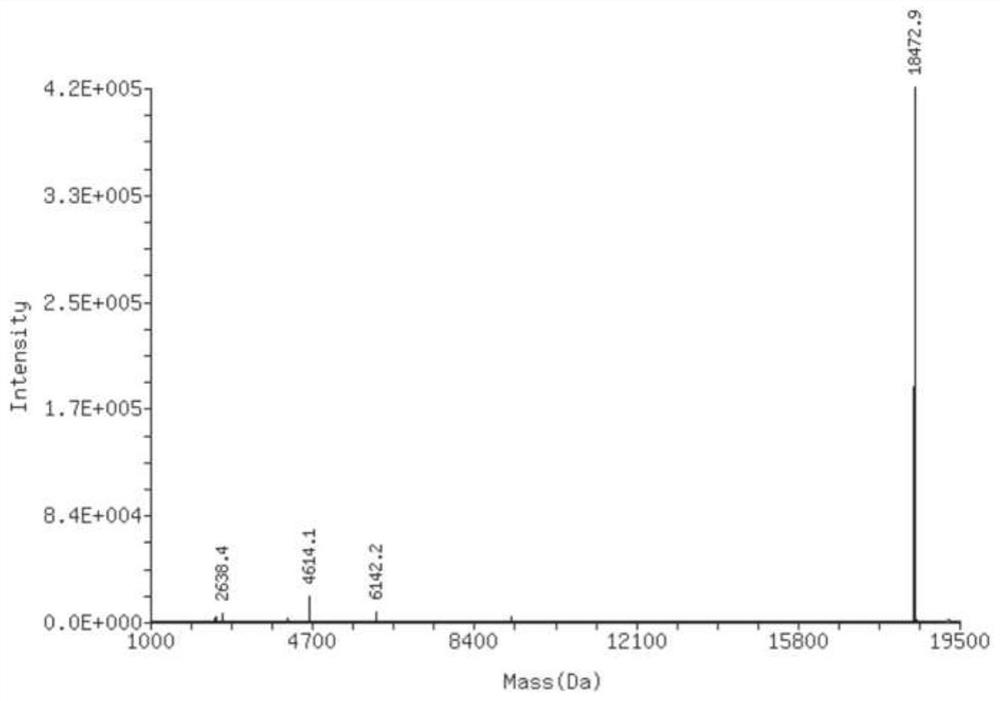
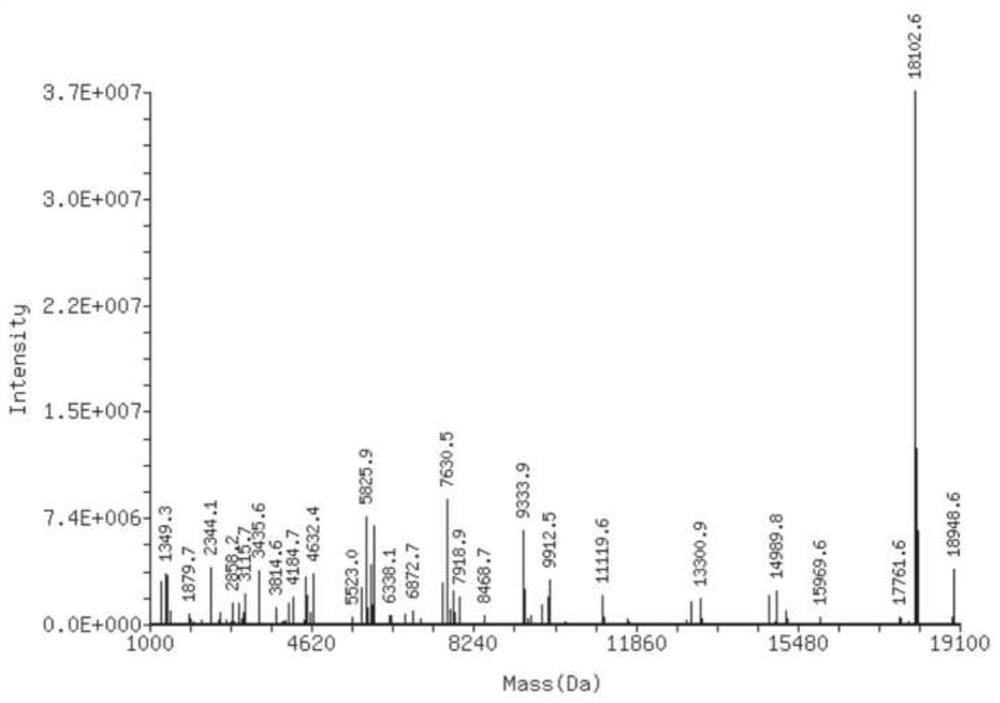
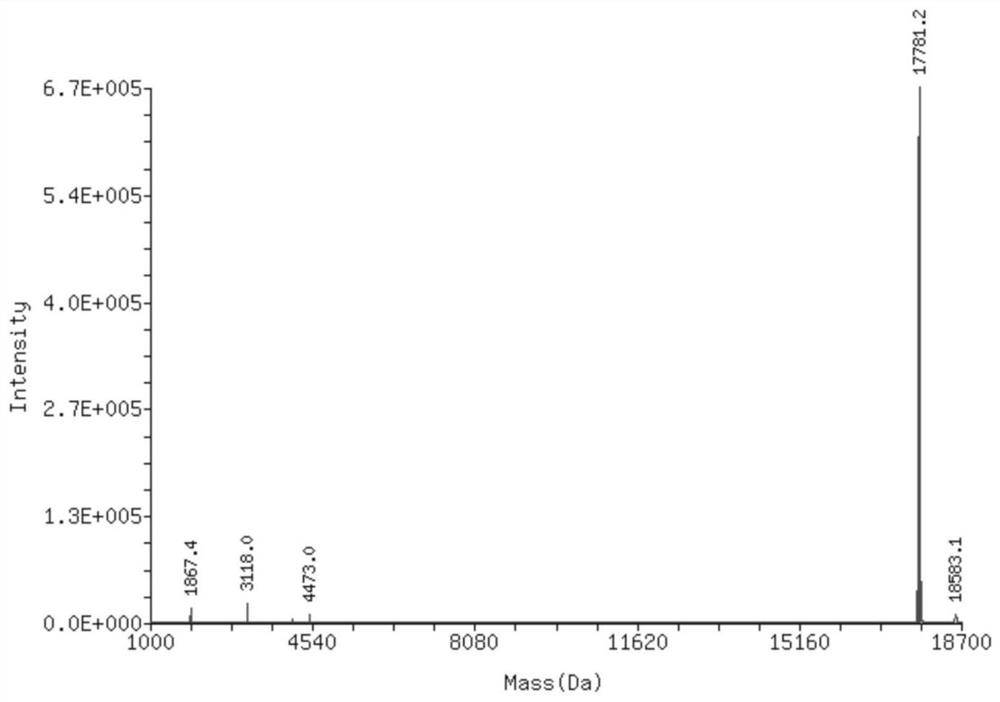
Clofarabine modified oligonucleotide
An oligonucleotide, clofarabine technology, applied in the field of medicinal chemistry, can solve the problems of programmed cell death, damage to mitochondrial membrane integrity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] The second aspect of the present invention provides the preparation method of the clofarabine modified oligonucleotide provided by the first aspect of the present invention, comprising: passing the clofarabine phosphoramidite monomer and / or the gemcitabine phosphoramidite monomer through solid The phase synthesis method is connected with the nucleic acid aptamer fragment.
[0070] In the preparation method of the clofarabine-modified oligonucleotide provided by the present invention, the conditions of suitable solid-phase synthesis method should be known to those skilled in the art, for example, the reaction conditions of solid-phase synthesis can be It is the coupling reaction condition of natural bases such as A, T, C, and G. For another example, the reaction temperature of the solid phase synthesis method can be 15~35°C, 15~20°C, 20~25°C, 25~30°C, or 30~35°C; the reaction time can be 1~20 minutes, 1~2 minutes, 2-4 minutes, 4-6 minutes, 6-8 minutes, 8-10 minutes, 10-...
Embodiment 1
[0138] Preparation of clofarabine phosphoramidite monomer:
[0139] 1) Add clofarabine (1 equivalent) to a one-mouth bottle, add 20 mL of N,N-dimethylformamide, imidazole (15 equivalents), and add tert-butyldimethylsilyl chloride (3.2 equivalents) to the solution at room temperature ). The mixture was stirred for 12h. The solvent was removed in vacuo using a rotary evaporator, and separation and purification were carried out by silica gel column chromatography to obtain TBDMS-clofarabine (99%) as a white solid. 1 H NMR (400MHz, Chloroform-d) δ8.05 (d, J = 2.6Hz, 1H), 6.51 (s, 2H), 6.43 (dd, J = 17.5, 3.9Hz, 1H), 4.99 (dt, J = 52.1,2.9Hz,1H),4.61(dt,J=18.0,2.9Hz,1H),3.94(q,J=4.5Hz,1H),3.84(d,J=4.6Hz,2H),0.92(d, J=3.8Hz,18H),0.14(d,J=1.9Hz,6H),0.10(s,6H)ppm.
[0140] The reaction formula of clofarabine reacting with tert-butyldimethylsilyl chloride (TBDMSCl) in N,N-dimethylformamide (DMF) under the effect of imidazole (imidazole) is as follows:
[0141]
[0142] 2) Add T...
Embodiment 2
[0155] Preparation of gemcitabine phosphoramidite monomer:
[0156] 1) Add gemcitabine (710 mg, 2.7 mmol) and 20 mL DMF to a 100 mL single-necked round bottom flask, and add acetic anhydride (280 μL, 2.96 mmol) to the solution at room temperature. N 2 The mixture was stirred overnight under protection. The solvent DMF was removed in vacuo using a rotary evaporator, the residue was mixed with silica gel, and separated and purified by silica gel column chromatography to obtain Ac-gemcitabine (616 mg, 75%) as a white solid. 1 H NMR (400MHz, DMSO-d 6 )δ11.02(s,1H),8.24(d,J=7.6Hz,1H),7.25(d,J=7.6Hz,1H),6.33(d,J=6.6Hz,1H),6.17(t, J=7.4Hz, 1H), 5.31(t, J=5.4Hz, 1H), 4.24-4.14(m, 1H), 4.11(q, J=5.3Hz, 1H), 3.89(dt, J=8.5, 3.0 Hz,1H),3.80(ddd,J=12.7,5.2,2.4Hz,1H),3.65(ddd,J=12.7,5.9,3.6Hz,1H),3.16(d,J=5.3Hz,2H),2.11 (s,3H)ppm.
[0157] Gemcitabine and acetic anhydride (Ac 2 O) in N, the reaction formula of N-dimethylformamide (DMF) reaction is as follows:
[0158]
[0159] 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com