Preparation and application of biodegradable functional hyperbranched polycarbonate compound
A polycarbonate and compound technology, applied in the field of preparation of functional hyperbranched polycarbonate compounds, can solve problems such as low yield and complex synthetic route, and achieve simple and convenient synthetic process, great application prospects, and good biocompatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 synthesizes hyperbranched polycarbonate dPC (HAC: AEC 2: 1) containing azide group
[0021] One-pot Synthesis of Hyperbranched Polycarbonate
[0022]
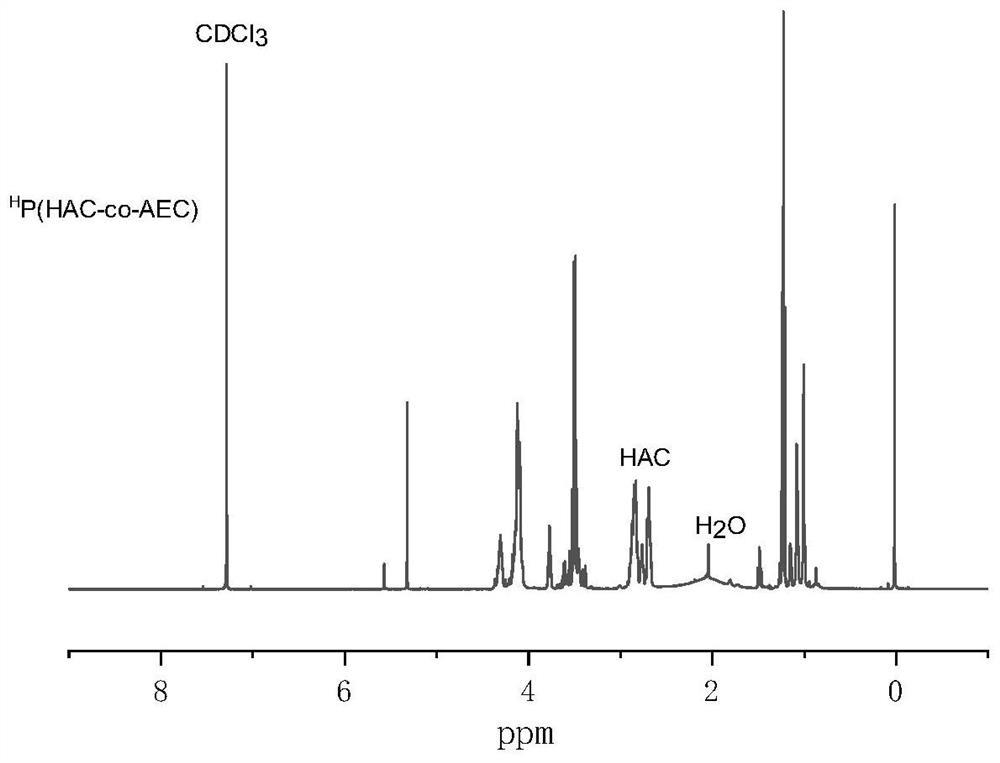
[0023] hyperbranched H Synthesis of P(HAC-co-AEC): In a glove box, AC (1g, 5mmol) monomer was dissolved in 10mL of dichloromethane, added to a closed reactor, and then mercaptoethanol ME (197.27mg, 2.525 mmol) and catalytic amount of triethylamine, reacted at room temperature for 4h. After the reaction time finishes, add the AEC monomer (427.9mg, 2.5mmol) solution that is dissolved in 4mL dichloromethane, then directly add catalyst DBU in the reaction solution, then the reactor is sealed, transfer out glove box, put into 50 The reaction was carried out in an oil bath at ℃ for 48 hours. After the reaction was completed, the reaction was terminated with 2 drops of glacial acetic acid, precipitated in glacial ether, the supernatant was discarded, the transparent oily viscous liquid at the bottom was collec...
Embodiment 2
[0024] Example 2 Synthesis of hyperbranched polycarbonate dPC (HAC: AEC 1: 1) containing azide group
[0025] hyperbranched H Synthesis of P(HAC-co-AEC): In the glove box, AC (500mg, 2.5mmol) monomer was dissolved in 8mL of dichloromethane, added to a closed reactor, and then mercaptoethanol ME (394.5mg, 5.05 mmol) and catalytic amount of triethylamine, reacted at room temperature for 4h. After the reaction time ended, a solution of AEC monomer (427.9 mg, 2.5 mmol) dissolved in 4 mL of dichloromethane was added, and then directly to the reaction solution, catalyst 1,8-diazabicycloundecene-7-ene ( DBU), then seal the reactor well, transfer it out of the glove box, put it into an oil bath at 50°C for 48 hours, stop the reaction with 2 drops of glacial acetic acid after the reaction, precipitate in glacial ether, discard the supernatant, collect Transparent oily viscous liquid at the bottom, vacuum dried to obtain the product H P(HAC-co-AEC). NMR results show that the ratio o...
Embodiment 3
[0026] Embodiment 3 synthesizes the hyperbranched polycarbonate dPC (HAC: AEC 1: 2) of azide group
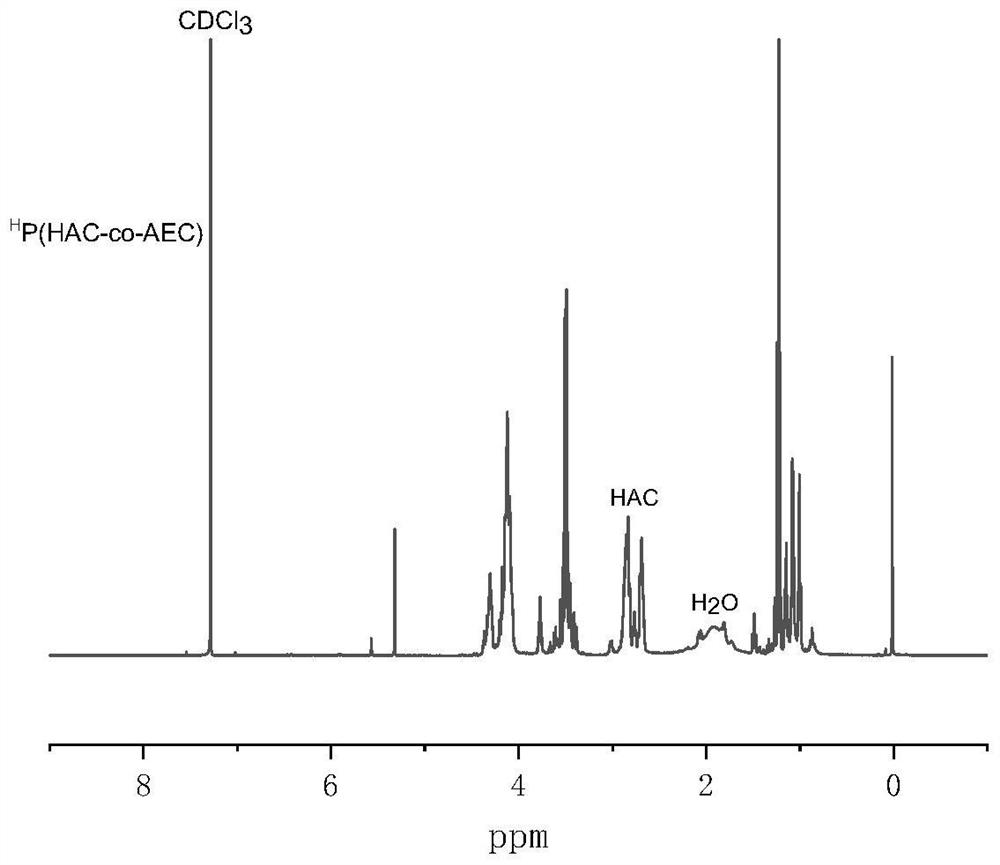
[0027] hyperbranched H Synthesis of P(HAC-co-AEC): In the glove box, AC (500mg, 2.5mmol) monomer was dissolved in 8mL of dichloromethane, added to a closed reactor, and then mercaptoethanol ME (394.5mg, 5.05 mmol) and catalytic amount of triethylamine, reacted at room temperature for 4h. After the reaction time finished, add the AEC monomer (855.8mg, 5mmol) solution that is dissolved in 8mL dichloromethane, then directly add catalyst 1,8-diazabicycloundec-7-ene (DBU ), then seal the reactor well, transfer it out of the glove box, and put it in an oil bath at 50°C for 48 hours. After the reaction, stop the reaction with 2 drops of glacial acetic acid, precipitate in glacial ether, discard the supernatant, and collect the bottom Transparent oily viscous liquid, vacuum dried to obtain the product H P(HAC-co-AEC). NMR results show that the ratio of HAC unit and AEC unit in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com