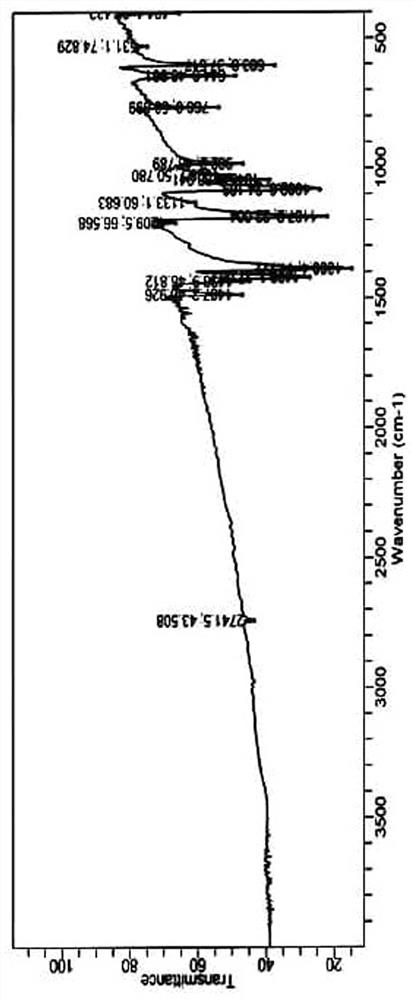
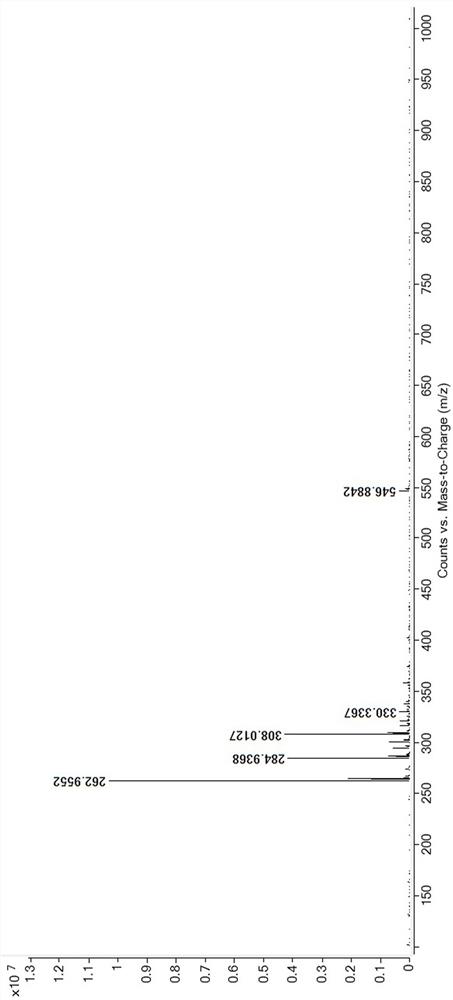
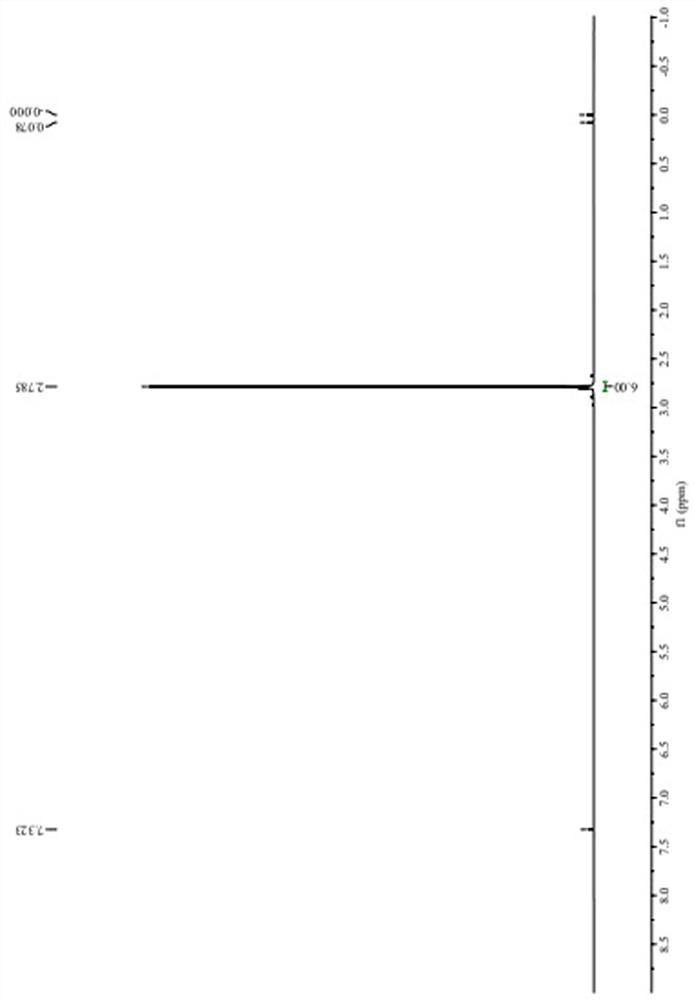
Preparation method of 2-methyl-5-sulfydryl-1, 3, 4-thiadiazole dimer
A technology of thiadiazole dimer and thiadiazole, which is applied in organic chemistry and other fields, can solve the problems that structural analysis and pharmacological research cannot be carried out, and achieve the effect of novel synthetic route, easy-to-obtain product and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 420g N,N-dimethylformamide into a 1000ml clean four-necked bottle, start stirring, add 100.0g methylmercaptothiadiazole when the temperature is lowered to 15°C, stir to dissolve, then add 310ml 30% hydrogen peroxide solution dropwise, The process control temperature does not exceed 35°C. After observing crystal precipitation, stop adding the hydrogen peroxide solution dropwise, control the temperature at 30-35°C and stir the crystal for 30 minutes, then continue to add the remaining hydrogen peroxide solution dropwise. After the dropwise addition, control the temperature Stir and react at 30-35°C for 30 minutes, then lower the temperature to 5°C, grow crystals for 30 minutes, and then filter. The filter cake is dried under reduced pressure in an oven at 60°C for 3 hours, and 59.5 g of the target product is obtained after reaching a constant weight. After testing, this example The purity (HPLC) of the target product methylmercaptothiadiazole dimer is 97.9%. The targe...
Embodiment 2
[0041] Add 150g N,N-dimethylacetamide into a 500ml clean four-necked bottle and start stirring. When the temperature is lowered to 13°C, add 26.5g methylmercaptothiadiazole. Oxyacetic acid solution, the process control temperature does not exceed 35°C. After observing that crystals are precipitated, stop dripping the peroxyacetic acid solution, control the temperature at 30-35°C and stir the crystal for 35 minutes, and then continue to drop the remaining peracetic acid solution until the addition is complete. Finally, control the temperature at 30-35°C, stir and react for 30 minutes, then cool down to 9°C, grow crystals for 30 minutes, and then filter. The filter cake is dried in an oven at 50°C under reduced pressure for 3 hours, and 16.9 g of the target product is obtained after reaching a constant weight. , the purity (HPLC) of the target product methylmercaptothiadiazole dimer in this example is 98.4%.
Embodiment 3
[0043] Add 180g dimethyl sulfoxide into a 500ml clean four-necked bottle and start stirring. When the temperature is lowered to 10°C, add 22.0g methylmercaptothiadiazole. After stirring to dissolve, add dropwise 50ml of 30% hydrogen peroxide solution , the process control temperature does not exceed 35°C. After observing that crystals are precipitated, stop adding hydrogen peroxide solution, control the temperature at 30-35°C and stir and grow crystals for 30 minutes, and then continue to add the remaining hydrogen peroxide solution dropwise. After the dropwise addition, control Stir and react at 30-35°C for 30 minutes, then lower the temperature to 7°C, grow crystals for 30 minutes, and then filter. The filter cake is dried in an oven at 70°C for 3 hours under reduced pressure, and 12.4 g of the target product is obtained after reaching a constant weight. After testing, this implementation The purity (HPLC) of the target product methylmercaptothiadiazole dimer is 98.5%.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com