Method for preparing m-nitrobenzaldehyde by using microchannel reactor
A micro-channel reactor, nitrobenzaldehyde technology, applied in chemical instruments and methods, chemical/physical/physical-chemical reactors, preparation of organic compounds, etc. and other problems, to achieve the effect of improving purity and yield, speeding up reaction rate, and continuous reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
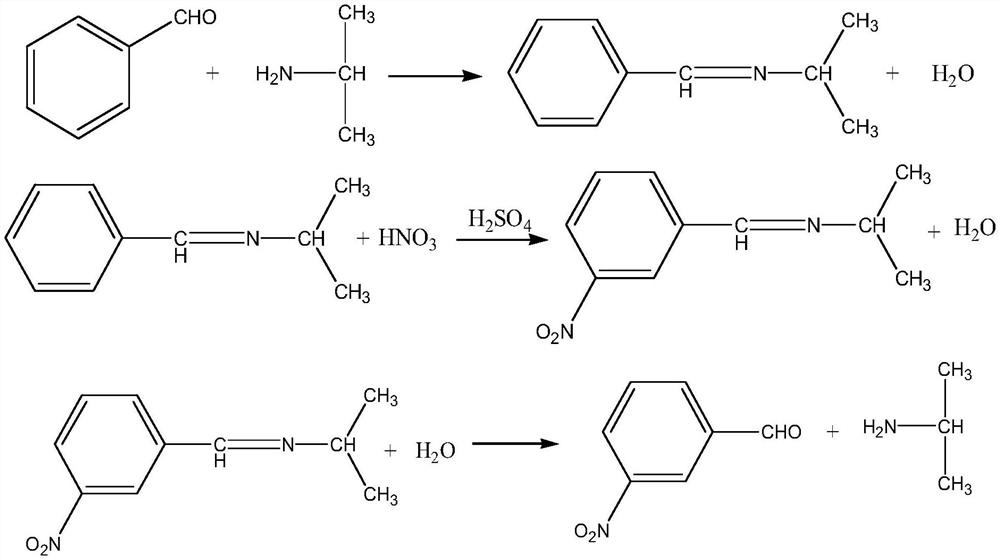
[0042] The present embodiment provides a kind of method utilizing microchannel reactor to prepare m-nitrobenzaldehyde, and concrete steps are as follows:
[0043] condensation
[0044] Use a metering pump to pump benzaldehyde and isopropylamine into the first microchannel reactor respectively to carry out condensation reaction to generate Schiff base, and control the temperature of the reaction system in the first microchannel reactor to be 10-20°C, wherein benzene The pumping flow rate of formaldehyde is 200mL / min, and the pumping flow rate of isopropylamine is 166mL / min. During the pumping process, a balance is used to check the amount of pumped benzaldehyde and isopropylamine.
[0045] nitrification
[0046] Mixed acid configuration: after mixing nitric acid with a mass fraction of 98% and sulfuric acid with a mass fraction of 98% according to a mass ratio of 1:9, cool to room temperature and set aside;
[0047] The product Schiff base obtained after the condensation reac...
Embodiment 2
[0053] The method that utilizes microchannel reactor to prepare m-nitrobenzaldehyde that this embodiment provides is similar to embodiment 1, and difference only is that the flow rate of each material is different, and the flow rate of benzaldehyde is 300mL / min in the present embodiment, and the flow rate of isopropylamine The flow rate of Schiff base is 273mL / min, the flow rate of Schiff base is 573mL / min, and the flow rate of mixed acid is 1157mL / min.
[0054] The yield of the m-nitrobenzaldehyde product that present embodiment makes is 94.2%, and HPLC purity is 99.6%
Embodiment 3
[0056] The method that utilizes microchannel reactor to prepare m-nitrobenzaldehyde that this embodiment provides is similar to embodiment 1, and difference only is that the flow rate of each material is different, and the flow rate of benzaldehyde is 500mL / min in the present embodiment, and the flow rate of isopropylamine is 435mL / min, the flow rate of Schiff base is 935mL / min, and the flow rate of mixed acid is 1841mL / min.
[0057] The yield of the m-nitrobenzaldehyde product obtained in this embodiment is 92.5%, and the HPLC purity is 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com