Coumarosone fluorescent probe for detecting Cu < 2 + > as well as preparation method and application of coumarosone fluorescent probe
A fluorescent probe, coumarin technology, which is applied in the field of chemical analysis, can solve the problem that the structure design is not novel enough, and achieve the effect of strong anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation method and usage method of probe L
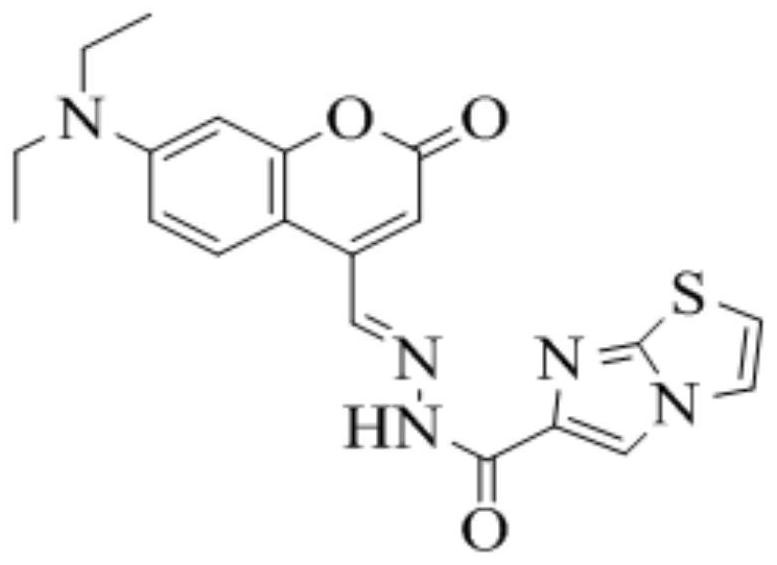
[0035] The present invention detects Cu 2+ The coumarin fluorescent probe named (E)-N'-(7-(diethylamino)-2-oxo-2H-benzopyran-4-ylmethylene)imidazo[2 , 1-b] thiazole-6-hydrazide, hereinafter or referred to as probe L, the structural formula is as follows figure 1 shown.
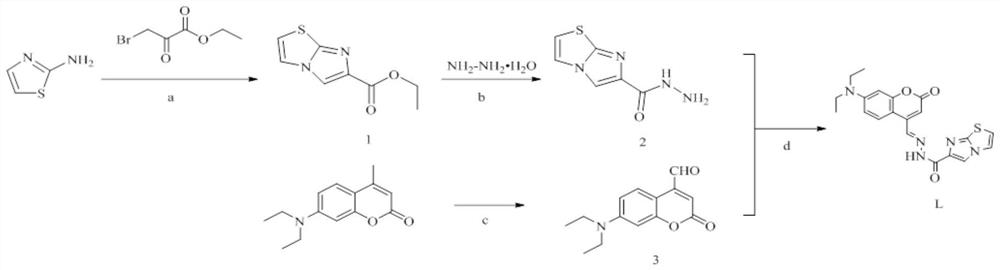
[0036] 1) The preparation method is as follows (the preparation reaction formula is as follows figure 2 shown):
[0037] Synthesis of ethyl imidazo[2,1-b]thiazole-6-carboxylate (1): Slowly add ethyl 3-bromopyruvate dropwise to 2-aminothiazole (1.00 g, 10 mmol) in tetrahydrofuran (100 mL) The ester (2.68g, 11mmol) was added dropwise for 5 minutes, and the color of the solution turned brown. After stirring at room temperature for 24 hours, a precipitate formed. The precipitate was filtered and rinsed three times with tetrahydrofuran to obtain a white powder. It was dissolved in 50 mL of absolute ethanol, refluxed for 4 h, filtered under ...
Embodiment 2
[0044] Embodiment 2: the recognition of metal ion by probe L
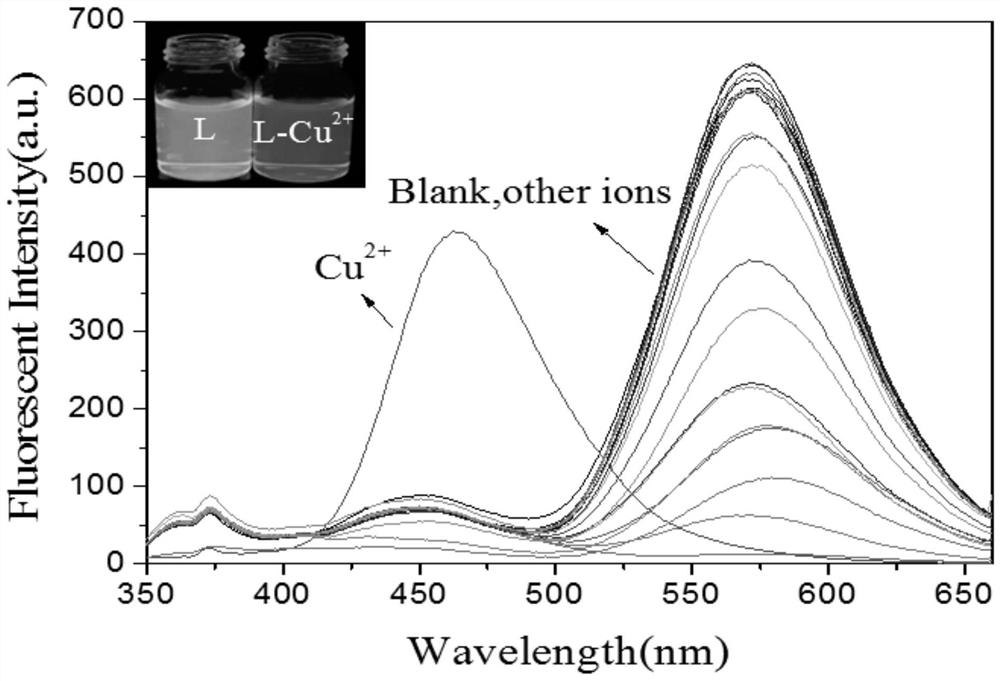
[0045] In order to study the recognition of different metal ions by probe L in acetonitrile solution, such as image 3 As shown, when the excitation wavelength is 335nm, the probe L (2.0×10 -5 mol L -1 ) has a maximum emission peak at 568nm, when adding 2×10 -4 mol L -1 Various metal ions (K + 、Na + , Li + 、Ag + , Ca 2+ , Mg 2+ , Zn 2+ 、Ba 2+ 、Co 2+ 、Cd 2+ , Sn 2+ 、Ni 2+ , Mn 2+ , Fe 2+ , Pb 2 + 、Sr 2+ , Fe 3+ 、Al 3+ 、Cr 3+ ), the fluorescence emission peak intensity was different, but the emission peak position did not change significantly; but adding Cu 2+ After that, the emission peak of probe L at 568nm shifted blue to 462nm, and the solution changed from orange fluorescence to blue fluorescence under the irradiation of 365nm ultraviolet lamp. This shows that the probe L on Cu 2+ highly selective recognition.
Embodiment 3
[0046] Example 3: Probe L and Cu 2+ UV Titration Experiment
[0047] To explore probe L and Cu 2+ The binding characteristics, carry out UV titration experiments, such as Figure 4 As shown, in acetonitrile solution, with Cu 2+ Concentration (0~20μmol·L -1 ), the probe L (2.0×10 -5 mol L -1 ) the absorption values at 256 and 497nm gradually increase, while the absorption values at 280, 316 and 428nm gradually decrease, and there are isoabsorbing points at 268 and 474nm, indicating that the probe L and Cu 2+ A stable complex is formed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com