Method for increasing yield of pichia pastoris foreign protein
A technology for exogenous protein and yield, applied in the field of bioengineering, to achieve the effect of good application value and increased expression of exogenous protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Construction of plasmids overexpressing heat stress-related genes
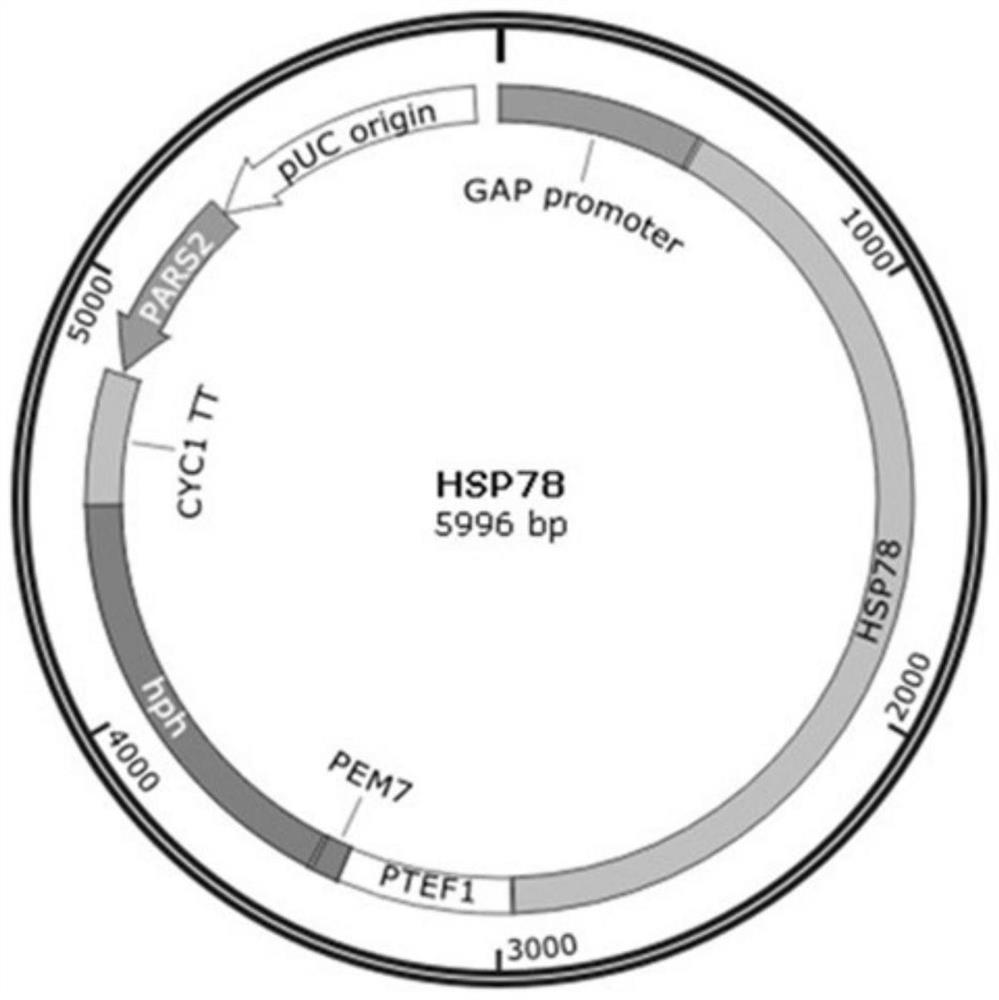
[0046] Using the Pichia pastoris genome as a template, the P1 and P2 primers were used to amplify the HSP78 gene fragment (the nucleotide sequence is shown in SEQID NO: 1); the vector pGAPZα was used as a template, and the P3 and P4 primers were used to amplify the vector fragment, using The seam cloning kit connects the two fragments into a circular plasmid and immediately transforms it into Escherichia coli competent cells. After sequencing verification, a positive transformant with correct sequencing is obtained, and the plasmid is extracted from the positive transformant to obtain an overexpression plasmid.
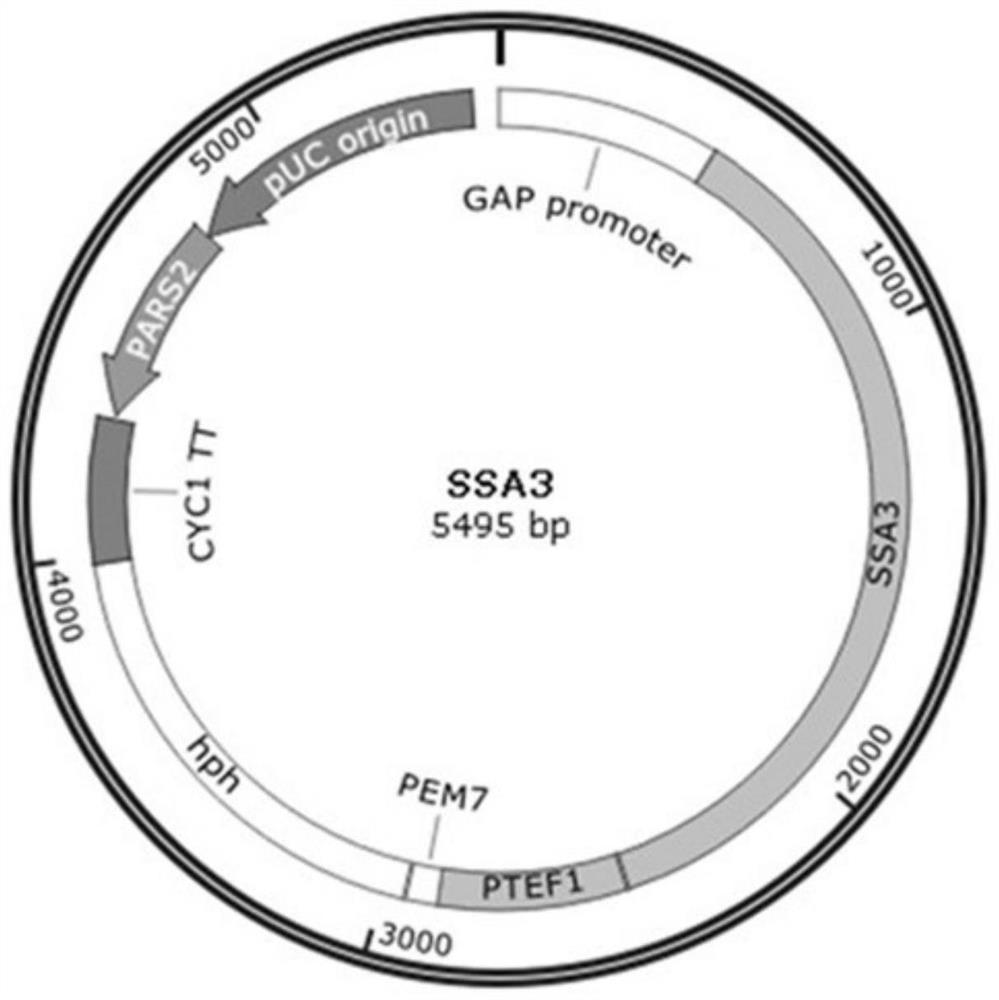
[0047] Using the Pichia pastoris genome as a template, primers P5 and P6 amplify the SSA3 gene fragment (nucleotide sequence shown in SEQ ID NO: 2), using the vector pGAPZα as a template, primers P7 and P8 amplify the vector fragment, and use seamless cloning The kit connects the t...
Embodiment 2
[0056] Example 2: Effects of overexpressing HSP78 and SSA3 on the expression of Pichia pastoris green fluorescent protein
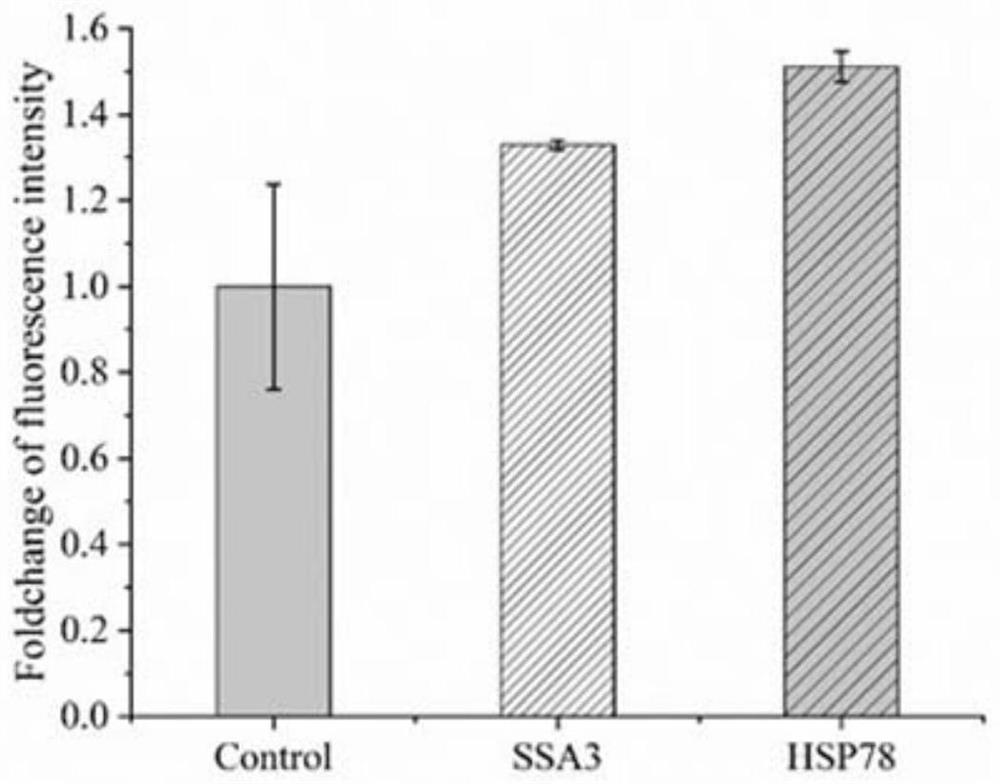
[0057] The overexpression plasmid constructed in Example 1 was linearized, and the linearized overexpression plasmid was transformed into P GAP Pichia pastoris expressing green fluorescent protein from the promoter (for the specific construction method of Pichia pastoris, see the literature Oxidativestress tolerance contributions to heterologous protein production in Pichiapastoris, published in 2021), constructing the overexpression of heat stress-related genes in Pichia pastoris Recombinant strains EGFP-SSA3 and EGFP-HSP78. It was determined by RT-qPCR that the gene transcription levels of SSA3 and HSP78 in the overexpression strain were increased by 4.7 times and 6.3 times, respectively, compared with those before the overexpression.
[0058] The constructed recombinant strains EGFP-SSA3 and EGFP-HSP78 were respectively inoculated in YPD medium for ...
Embodiment 3
[0059] Example 3: Verification of the fermentation expression of other foreign proteins by overexpressing HSP78 and SSA3
[0060] By transforming the linearized overexpression plasmids into lipase-expressing yeast respectively (for the specific construction method of Pichia pastoris expressing lipase, see the document Enhancement of lipase r27RCL production in Pichia pastoris by regulating gene dosage and co-expression with chaperoneprotein disulfide isomerase published in 2013 For the strain SRCL1) and phospholipase (for the specific construction method of Pichia expressing phospholipase, refer to the recombinant Pichia GS115 in the literature Streptomyces violeter Phospholipase A2: Expression in Pichia pastoris, Properties, and Application in Oil Degumming published in 2015 ) of Pichia pastoris competent, and construct a Pichia pastoris heat stress-related gene overexpression strain again. The single clone of the overexpressed strain was fermented in BMGY / BMMY medium. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com