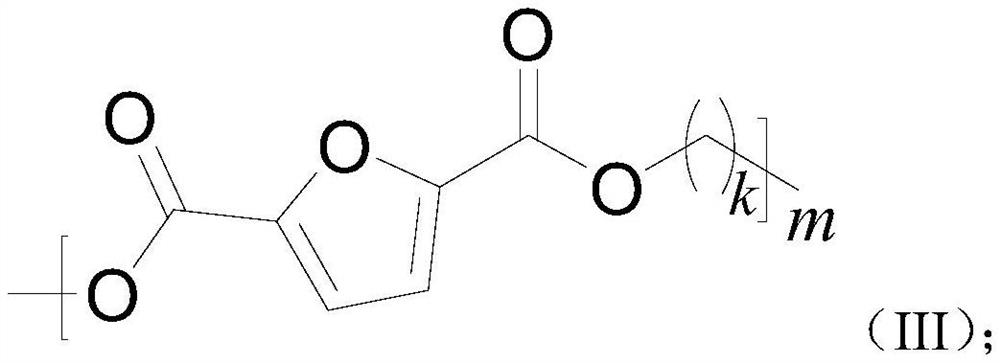
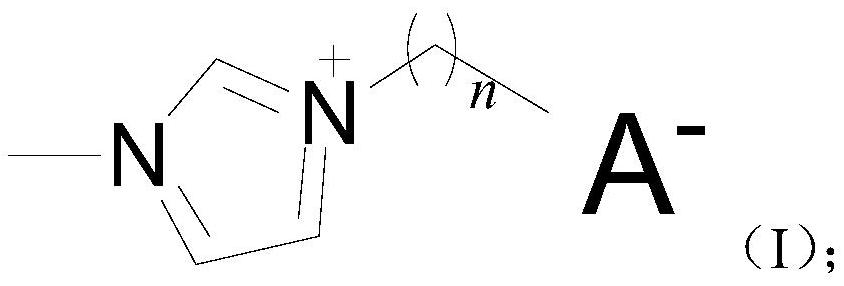Imidazole ionic liquid and application thereof in alcoholysis of 2, 5-furandicarboxylate
A technology of furan dicarboxylate and methylimidazole furan formate, which is applied in the fields of green, chemical production, plastics, and clean catalysis technology, can solve problems such as waste of resources, refractory degradation, and impact on the ecological environment, and reduce energy consumption , less catalyst consumption, improved catalytic activity and catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] The preparation method of imidazoles ionic liquid catalyst adopts two-step method, is example with 1-ethyl-3 methylimidazolium furoate, in the flask that reflux pipe, dropping funnel and stirring device are housed, add the N of 8.211g -Methylimidazole, slowly add 11.25g of bromoethane into the constantly stirring N-methylimidazole, keep the water bath above 30°C until the addition is complete, then keep for 72h, stop heating, cool down, pour out the excess bromoethane, and then vacuum the remaining bromoethane to obtain the initial product of 1-ethyl-3-methylimidazole bromide, which can be recrystallized; 15.10g potassium furoate and 19.10g bromide 1- Ethyl-3-methylimidazole was dissolved in 200mL of absolute ethanol, and then the solution of potassium furanate was added dropwise to the solution of 1-ethyl-3-methylimidazole bromide, and the reaction temperature was maintained at 50 °C, finally filter the reacted solution to remove potassium bromide, and distill off anhy...
Embodiment 1
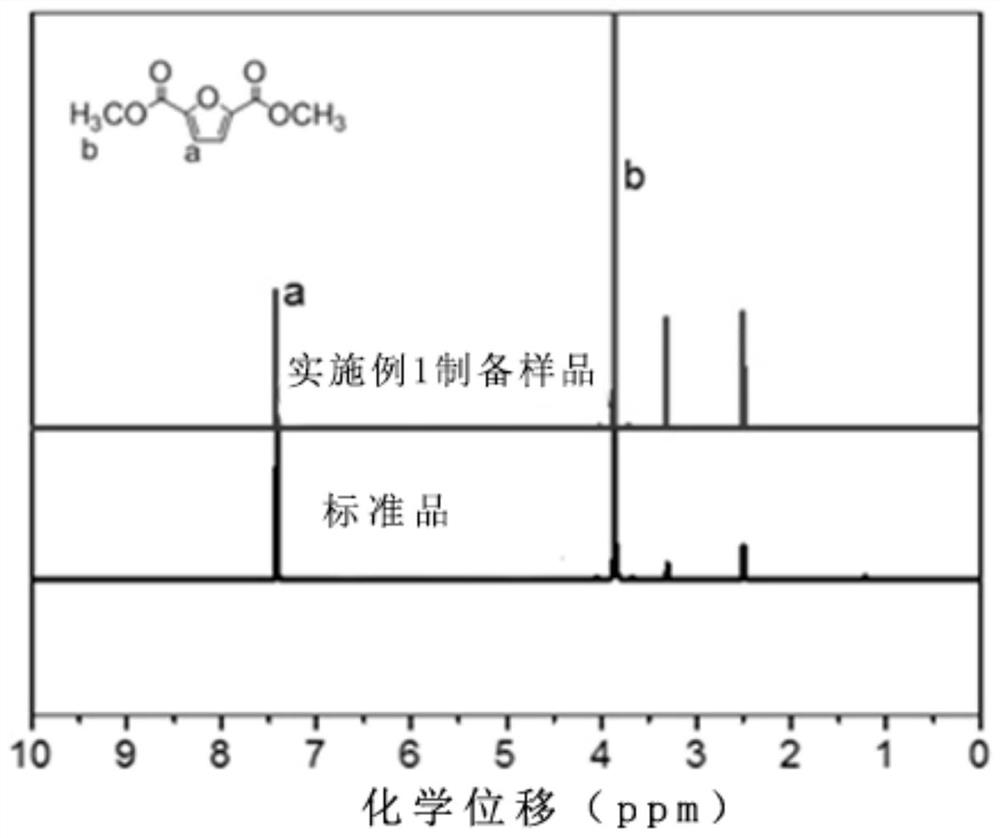
[0075] Take the reactants PEF, methanol and 1-butyl-3-methylimidazolium furoate and add them to a stainless steel autoclave with stirring and thermometer in sequence, wherein the mass of PEF is 5g, and the mass ratio of PEF to methanol is 1:1 , 1-butyl-3-methylimidazolium furoate accounts for 0.3% of the PEF mass; the reaction system is heated to 60°C in a water bath and kept for 3h; Filtration, washing and extraction steps to obtain pure dimethyl 2,5-furandicarboxylate, wherein, figure 1It is the NMR comparison chart of the product and the standard reagent dimethyl 2,5-furandicarboxylate, from which it can be determined that the product is dimethyl 2,5-furandicarboxylate. The calculated conversion rate of PEF is 100%, and the yield of dimethyl 2,5-furandicarboxylate is 97%.
Embodiment 2
[0077] Take the reactants PEF, methanol and 1-butyl-3-methylimidazolium furoate and add them to a stainless steel autoclave with stirring and thermometer in sequence, wherein the mass of PEF is 5g, and the mass ratio of PEF to methanol is 1:1 , 1-butyl-3-methylimidazolium furoate accounts for 0.5% of the PEF mass; the reaction system is heated to 60°C in a water bath and kept for 3h; Through the steps of filtration, washing and extraction, pure dimethyl 2,5-furandicarboxylate was obtained, the conversion rate of PEF was calculated to be 100%, and the yield of dimethyl 2,5-furandicarboxylate was 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com