Method for synthesizing xanthoxylin WGX-50 and derivatives thereof in one pot
A technology of WGX-50 and xanthanin, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of poor control of yield and purity, environmental hazards, improper handling, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment one, the preparation of catalyst system
[0036] The molecular sieve is activated at a high temperature of 140°C for 1-2 hours before use, and then the activated molecular sieve, catalyst and dehydrating agent are added to the reaction system before the reaction, and the catalytic system is obtained by replacing the inert gas.
Embodiment 2
[0037] Embodiment two, the preparation of xanthoxylin WGX-50 and derivative thereof
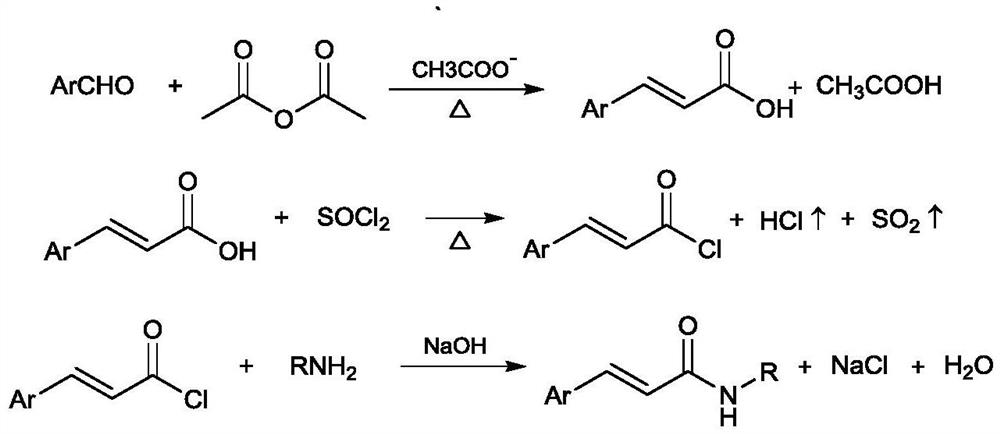
[0038] The chemical reaction formula is as follows:
[0039]
[0040] When substituent A is C 1 -C 3 For alkyl groups, take the synthesis of N-phenethyl cinnamic amide as an example.
[0041] Step 1-1, Weigh 5.00g (47.1mmol) of benzaldehyde and 50ml (293.3mmol) of n-butyl ether in a 100ml reaction bottle, install a condenser on the reaction bottle, and mix 6.69g (47.1mmol) of molecular sieve activated at 140°C with 6.69g (47.1mmol) ) phosphorus pentoxide and 1.09g (4.71mmol) zirconium chloride together form the catalyst system into the reaction flask, replace with inert gas nitrogen three times, discharge the air in the system, raise the temperature to 120 ° C, and then add 4.81g (47.1mmol) Acetic anhydride was slowly added to the system, reacted for 5 hours, and the acetic acid gas generated by the reaction was recovered through the condenser.
[0042] Step 1-2, remove the condenser o...
Embodiment 3
[0047] Embodiment three, the preparation of xanthoxylin WGX-50 and derivative thereof
[0048] The chemical reaction formula is as follows:
[0049]
[0050] When substituent A is selected from: -OCCH 3 , -OCOMe, C 1 -C 3 When alkoxy or phenyl, take the synthesis of N-(3,4-dimethoxyphenethyl)cinnamic amide as an example.
[0051] Step 1-1, Weigh 5.00g (47.1mmol) of benzaldehyde and 50ml (293.3mmol) of n-butyl ether in a 100ml reaction bottle, install a condenser on the reaction bottle, and mix 2.64g (47.1mmol) of molecular sieves activated at 140°C with 2.64g (47.1mmol) ) Calcium oxide and 1.09g (4.71mmol) zirconium chloride together form the catalytic system into the reaction flask, replace with inert gas nitrogen three times, discharge the air in the system, raise the temperature to 120 ° C, and then add 4.81g (47.1mmol) acetic anhydride Slowly add to the system, fully react for 6h, and the acetic acid gas generated is recovered through the condenser.
[0052] Step 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com