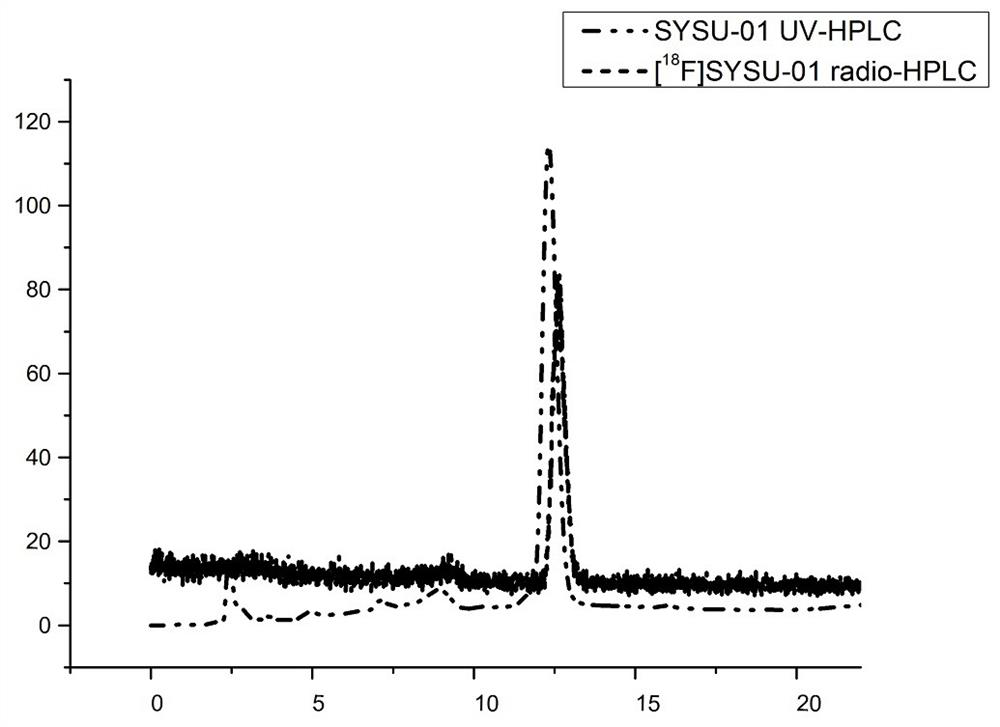
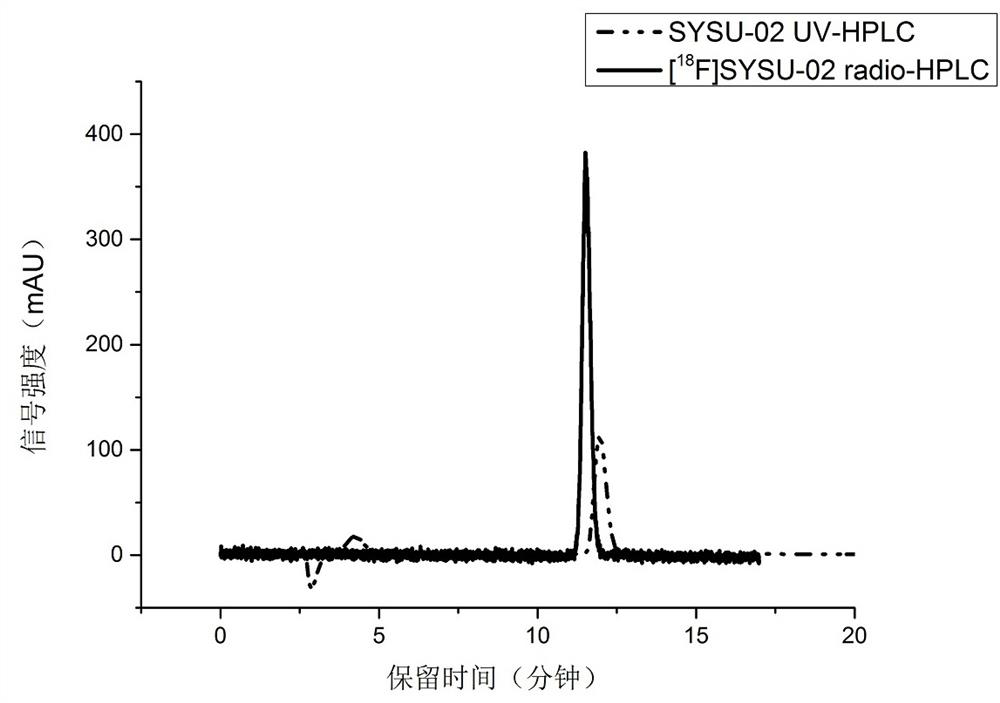
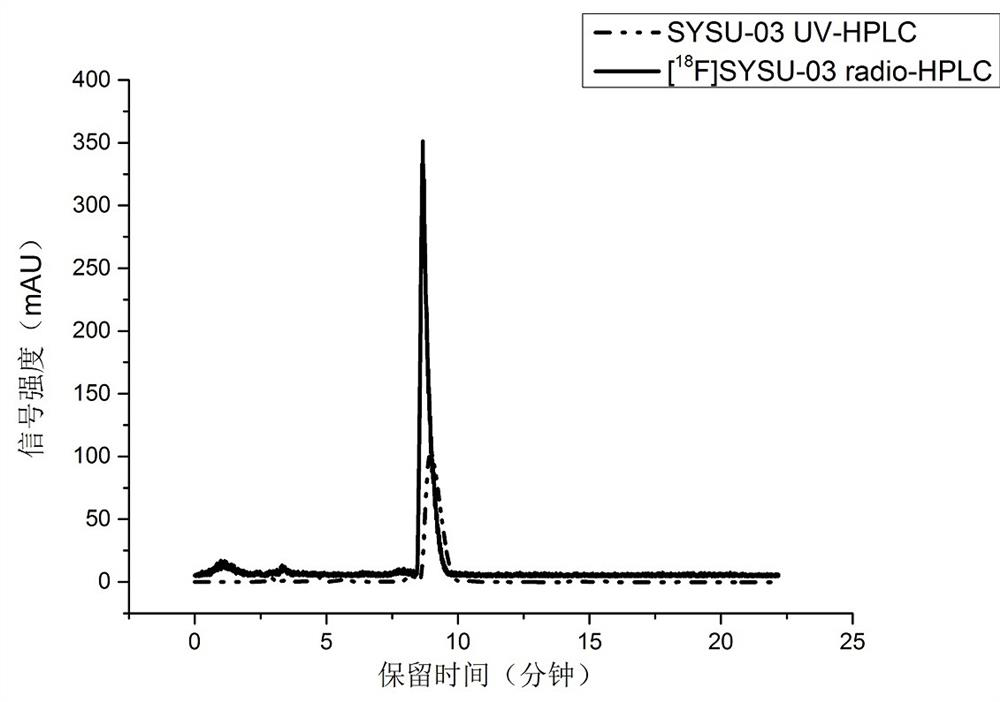
Preparation of fluorine-18 labeled targeted P2X7 receptor molecular probe and PET imaging application of fluorine-18 labeled targeted P2X7 receptor molecular probe in animal model
A technology of P2X7 and receptor molecules, applied in the field of nuclear medicine and imaging, can solve the problems of low yield, poor repeatability, no imaging research, etc., and achieve the effect of good specific binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] This example discloses a medical radioactive isotope-labeled P2X7 receptor targeting probe precursor, the structural formula of which is shown in Compound 1-1:
[0067]
[0068] (compound 1-1)
[0069]
[0070] The preparation method of the compound 1-1 is:
[0071] The compound (2-chloro-3-(trifluoromethyl)phenyl)formamide (500 mg, 2.39 mmol) and 8 mL of boron tribromide (1 M in CH 2 Cl 2 ), reacted at room temperature under nitrogen protection for one hour, then heated to reflux for two hours, monitored with gas chromatography-mass spectrometry after the reaction was completed, and separated and purified by column chromatography to obtain compound (3-(bromodifluoromethyl) -2-Chlorophenyl)methanamine (538 mg, 2 mmol). Then (3-(bromodifluoromethyl)-2-chlorophenyl)methanamine (500 mg, 1.86 mmol), (S)-1-methyl-5-oxopyrrolidine-3 -Carboxylic acid (266 mg, 1.86 mmol), 2-(7-azabenzotriazole)- N,N,N',N' - Tetramethyluronium hexafluorophosphate (HATU) (848 mg, 2.23...
Embodiment 2
[0081] This example discloses a medical radioactive isotope-labeled P2X7 receptor targeting probe precursor, the structural formula of which is shown in compound 1-2:
[0082]
[0083] (compound 1-2)
[0084]
[0085] The preparation method of the compound 1-2 is:
[0086] The compound (2-chloro-3-(trifluoromethyl)phenyl)formamide (750 mg, 3.59 mmol) and 12 mL of boron tribromide (1 M in CH 2 Cl 2 ), reacted at room temperature under nitrogen protection for one hour, then heated to reflux for two hours, monitored with gas chromatography-mass spectrometry after the reaction was completed, and separated and purified by column chromatography to obtain compound (3-(bromodifluoromethyl) -2-Chlorophenyl)methanamine (807 mg, 3 mmol). Then (3-(bromodifluoromethyl)-2-chlorophenyl)methanamine (807 mg, 3 mmol), (S)-1-methyl-5-oxopyrrolidine-2 -Carboxylic acid (430 mg, 3 mmol), 2-(7-azabenzotriazole)- N,N,N',N' - Tetramethyluronium hexafluorophosphate (HATU) (1.369 g, 3.6 mmol...
Embodiment 3
[0096] This example discloses a medical radioactive isotope-labeled P2X7 receptor targeting probe precursor, the structural formula of which is shown in Compound 1-3:
[0097]
[0098] (Compounds 1-3)
[0099]
[0100] The preparation method of the compound 1-3 is:
[0101] 2,4,6-Trimethylphenylboronic acid (285 mg, 1.7 mmol) was dissolved in dichloromethane (5 mL). The solution was cooled to 0°C and BF was added 3 •Et 2 O (225 µL, 1.8 mmol), and the mixture was stirred for 5 min. Then (2-iodo-3-(trifluoromethyl)phenyl)formamide (512 mg.1.7 mmol) was added dropwise as a solution in dichloromethane (3 mL). The mixture was slowly warmed to room temperature and stirred for 3.5 hours. By adding saturated NaBF 4 (aqueous solution, 3 mL) to quench the reaction. After stirring vigorously for 4 h, the aqueous layer was extracted with dichloromethane (2 x 50 mL). The combined organic layers were in Na 2 SO 4 Dry over, filter, and concentrate in vacuo. The residue was p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com