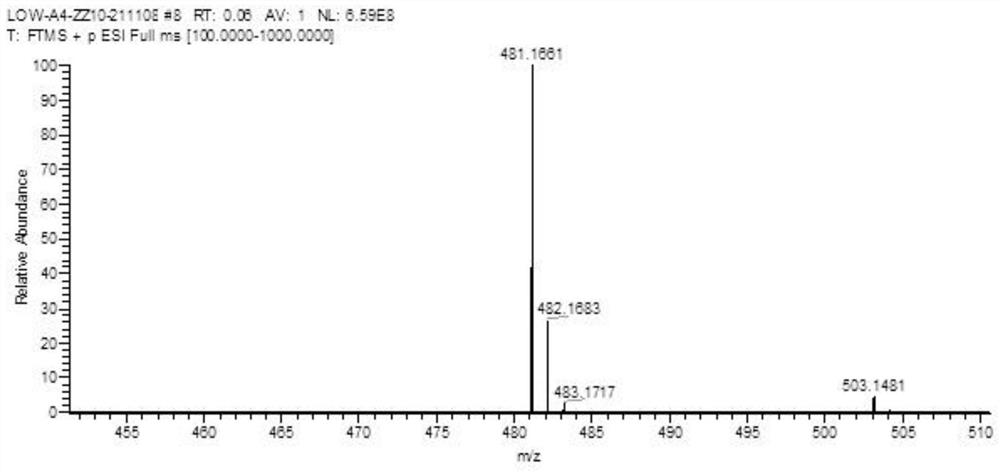
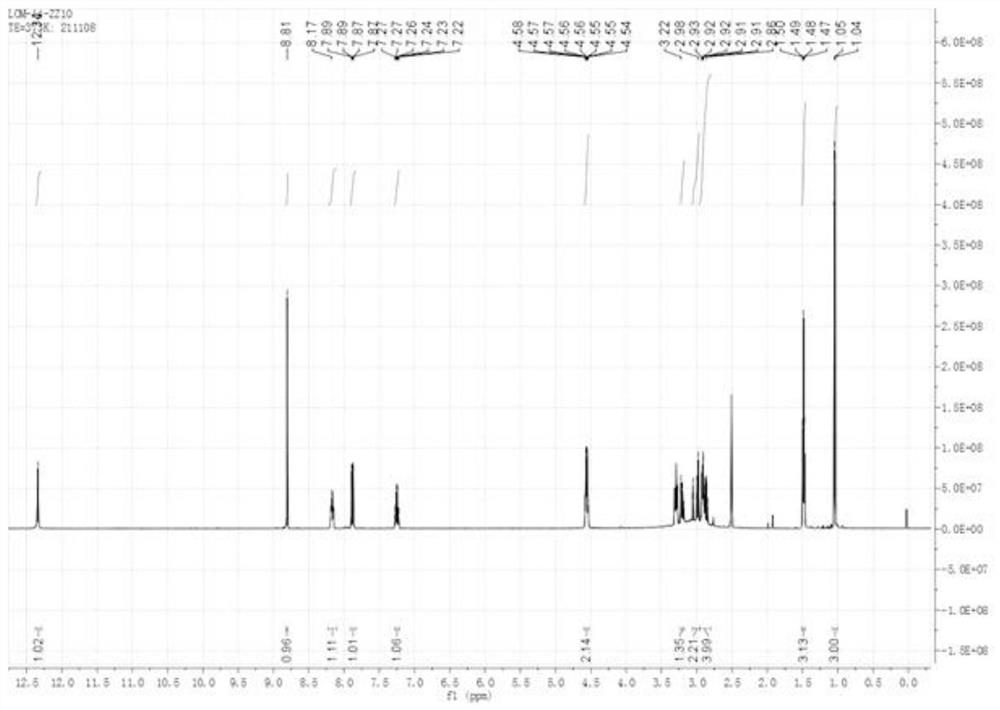
Lomefloxacin hydrochloride impurity and preparation method thereof
A technology for lomefloxacin hydrochloride and impurities, which is applied in the field of lomefloxacin hydrochloride impurities and their preparation, can solve problems such as synthesis without relevant content, and achieve the effects of improving accuracy, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of Compound II:
[0028] Add 135g of raw material I and 198.5g of EMME to a 2L reaction flask, stir and heat up to 115°C for 3 hours, add 0.8L of diphenyl ether to the feed liquid, stir and heat up to 243°C for 1h, cool down to 55°C, add 200mL THF, and stir It was filtered and dried to obtain 192.6 g of compound II. Yield 77%.
[0029] Preparation of compound III:
[0030] Add 165g of compound II and 1.3L of DMF to a 3L reaction flask, stir and heat up to 110°C, add 160g of potassium carbonate and 210g of bromoethane, stir and react at 115°C for 3h, after cooling down, add 1.3L of methyl tert-butyl ether and 1.3 L of purified water was stirred for crystallization, filtered, and dried to obtain 166.2 g of compound III. Yield 89%.
[0031] Preparation of Compound IV:
[0032] Add 145g of compound III and 900mL of glacial acetic acid into a 3L reaction flask, stir to raise the temperature to dissolve, add 300g of concentrated hydrochloric acid, stir and re...
Embodiment 2
[0038] Preparation of Compound II:
[0039] Add 140g of raw material I and 205.8g of EMME to a 2L reaction flask, stir and heat up to 112°C for 3 hours, add 1.6L of diphenyl ether to the feed liquid, stir and heat up to 243°C for 1h, cool down to 55°C, add 400mL of tetrahydrofuran, stir It was filtered and dried to obtain 199.2 g of compound II. Yield 76.8%.
[0040] Preparation of compound III:
[0041] Add 170g of compound II and 1.4L of DMF to a 3L reaction flask, stir and heat up to 110°C, add 165g of potassium carbonate and 212g of bromoethane, stir and react at 115°C for 3h, after cooling down, add 1.4L of methyl tert-butyl ether and 1.4 L of purified water was stirred for crystallization, filtered, and dried to obtain 167.4 g of compound III. Yield 88%.
[0042] Preparation of Compound IV:
[0043] Add 150g of compound III and 800mL of glacial acetic acid into a 3L reaction flask, stir to dissolve the liquid, add 305g of concentrated hydrochloric acid, stir and rea...
Embodiment 3
[0049] Preparation of Compound II:
[0050] Add 145g of raw material I and 213.2g of EMME to a 2L reaction flask, stir and heat up to 120°C for 3 hours, add 2L of diphenyl ether to the feed solution, stir and heat up to 243°C for 1h, cool down to 55°C, add 500mL of tetrahydrofuran, stir and filter , and dried to obtain 210 g of compound II. Yield 78.2%.
[0051] Preparation of compound III:
[0052] Add 175g of compound II and 1.42L of DMF to a 3L reaction flask, stir and heat up to 110°C, add 170g of potassium carbonate and 213.4g of bromoethane, stir and react at 115°C for 3h, add 1.42L of methyl tert-butyl ether and 1.42L of purified water was stirred and crystallized, filtered and dried to obtain 169.2g of compound III. Yield 87.5%.
[0053] Preparation of Compound IV:
[0054]Add 155g of compound III and 700mL of glacial acetic acid into a 3L reaction flask, stir to dissolve the liquid, add 315g of concentrated hydrochloric acid, stir and react at 104°C for 3h, cool ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com