Beta-chloro tetra-substituted alkenyl sulfone compound and synthesis method thereof
A synthetic method and compound technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., to achieve the effects of wide substrate applicability, easy operation, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
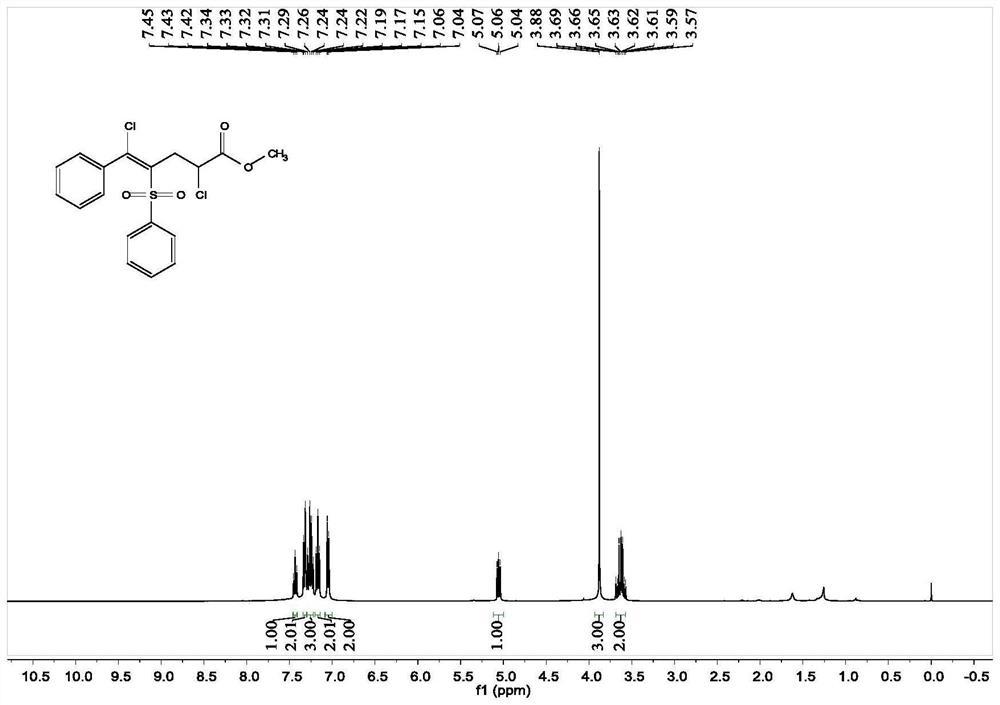
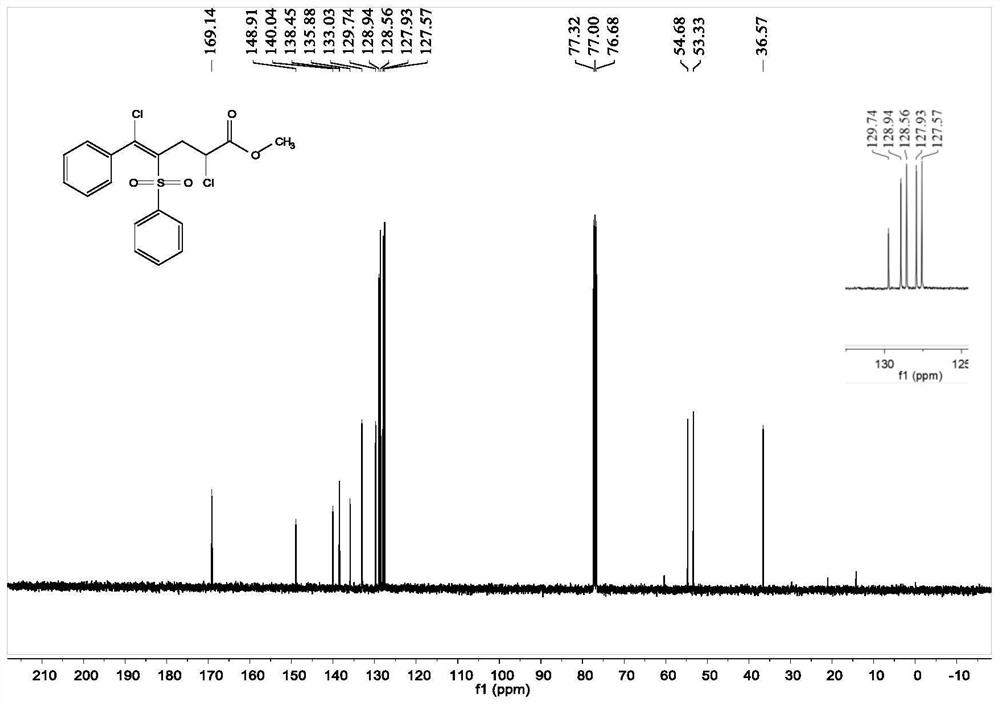
[0043]Add 0.1 mmol of [(phenylethynyl) sulfone] benzene, 0.3 mmol of methyl acrylate, 0.3 mmol of copper chloride, 0.01 mmol of palladium chloride and 1 ml of acetonitrile solvent in the reaction tube, and rotate at 40 degrees Celsius The reaction was stirred at 500 rpm for 12 hours, and the stirring was stopped. Add 5 mL of saturated sodium chloride solution, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, concentrate under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The chromatographic eluent was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 15:1, and the yield was 89%.
[0044] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows figure 1 and figure 2 shown; the structural characterization data are shown below:
[0045] 1 H-NMR (400MHz, CDCl 3 )δ7...
Embodiment 2
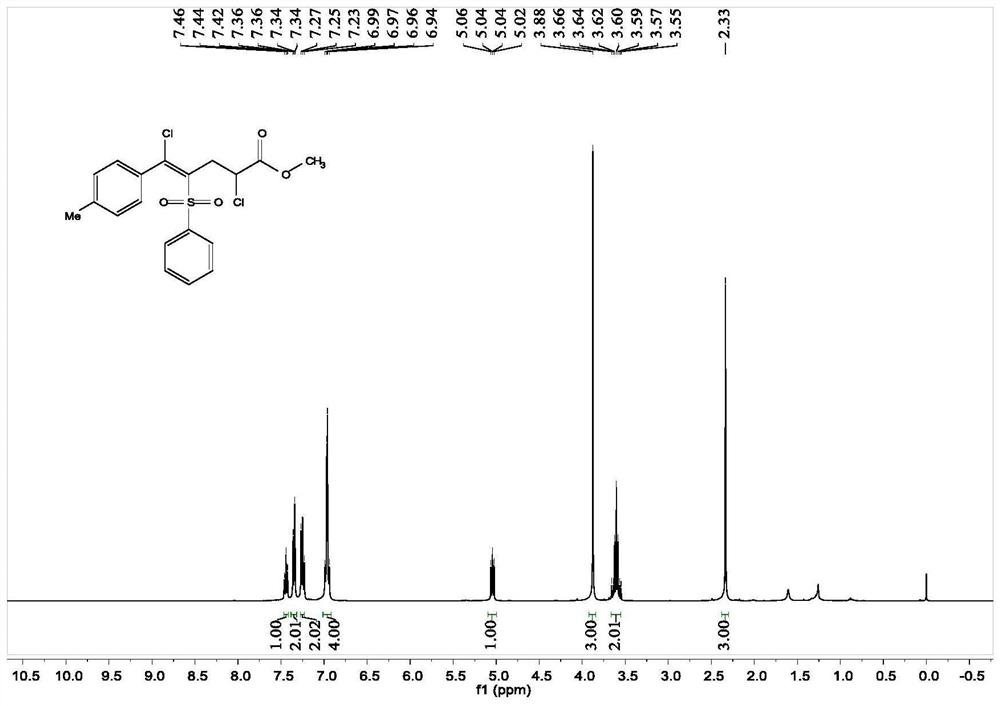
[0052] Add 0.1 mmol 1-methyl-4-[(phenylsulfonyl)ethynyl]benzene, 0.3 mmol methyl acrylate, 0.3 mmol copper chloride, 0.01 mmol palladium chloride and 1 mL acetonitrile to the reaction tube Solvent, stirred and reacted at 45 degrees Celsius at 500 rpm for 12 hours, and stopped stirring. Add 5 mL of saturated sodium chloride solution, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, concentrate under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The chromatographic eluent was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 15:1, and the yield was 86%.
[0053] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows image 3 and Figure 4 shown; the structural characterization data are shown below:
[0054] 1 H-NMR (400MHz, CDCl 3 )δ7.44(t, J=7.4Hz, 1H), 7.35(dd...
Embodiment 3
[0061] Add 0.1 mmol 1-methoxy-4-[(phenylsulfonyl)ethynyl]benzene, 0.3 mmol methyl acrylate, 0.3 mmol copper chloride, 0.01 mmol palladium chloride and 1 ml Acetonitrile solvent, stirred and reacted at 500 rpm at 45 degrees Celsius for 12 hours, and stopped stirring. Add 5 mL of saturated sodium chloride solution, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, concentrate under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The chromatographic eluent was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 5:1, and the yield was 60%.
[0062] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows Figure 5 and Figure 6 shown; the structural characterization data are shown below:
[0063] 1 H NMR (400MHz, CDCl 3 )δ7.44(t, J=6.7Hz, 1H), 7.36(d, J=9.7Hz, 2H), 7.28(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com