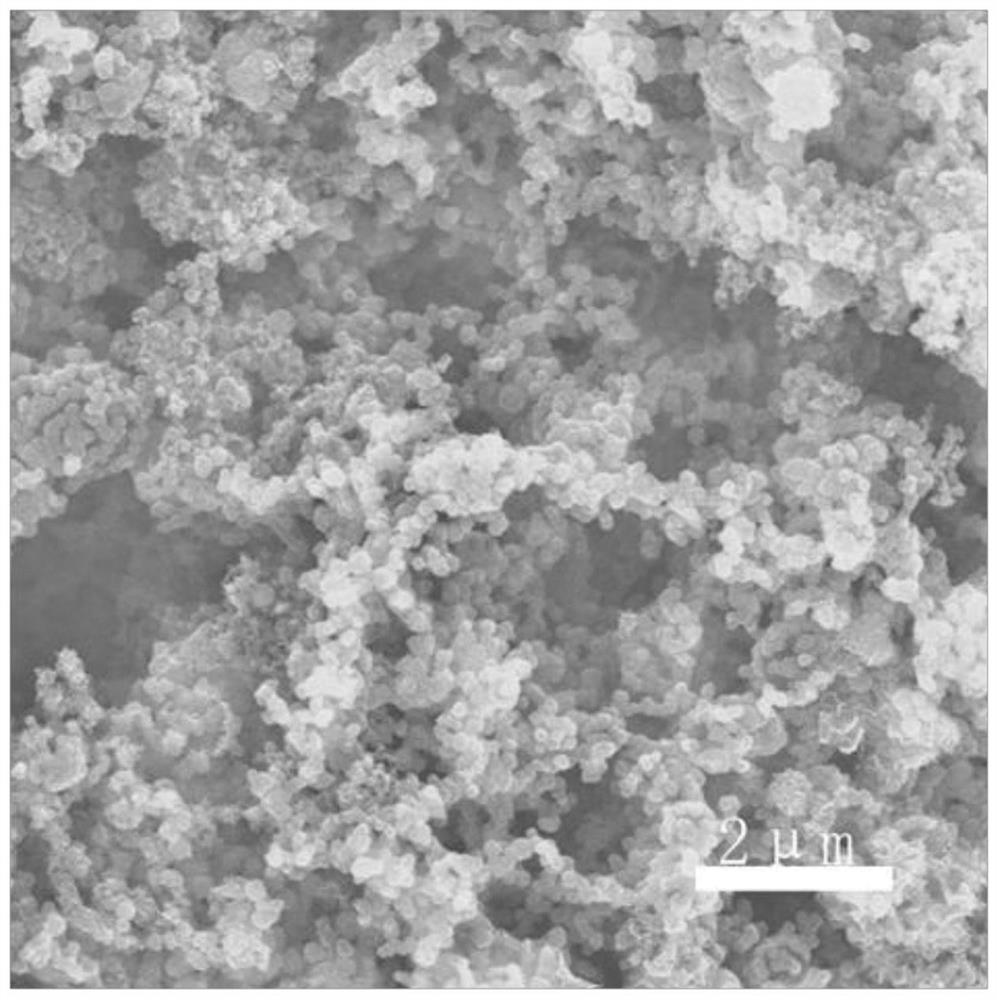
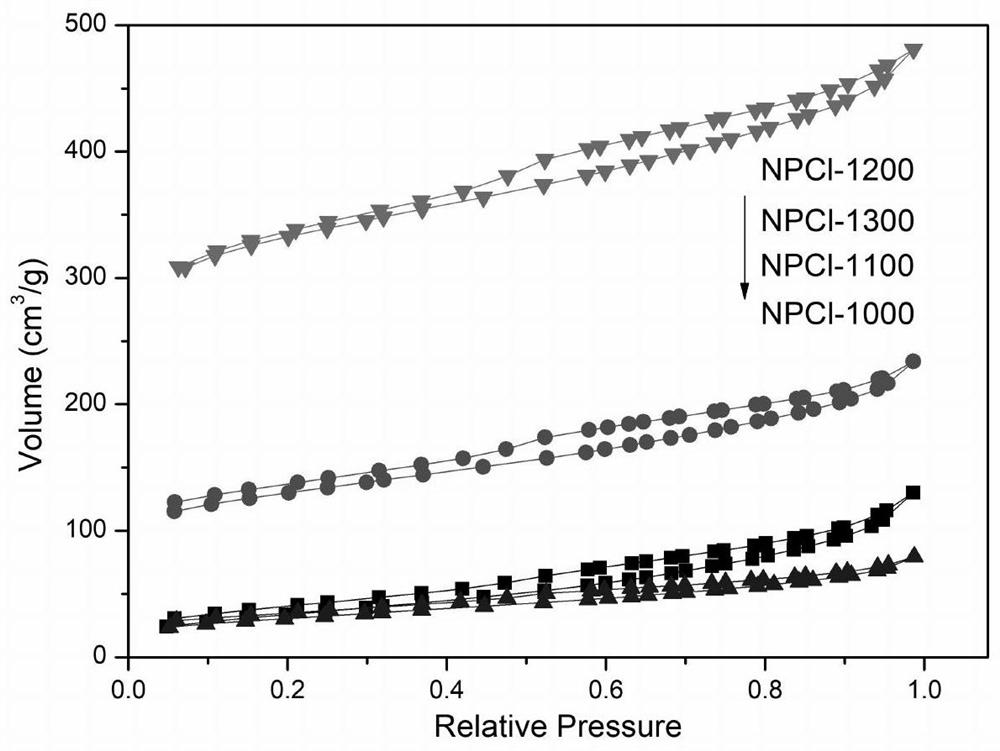
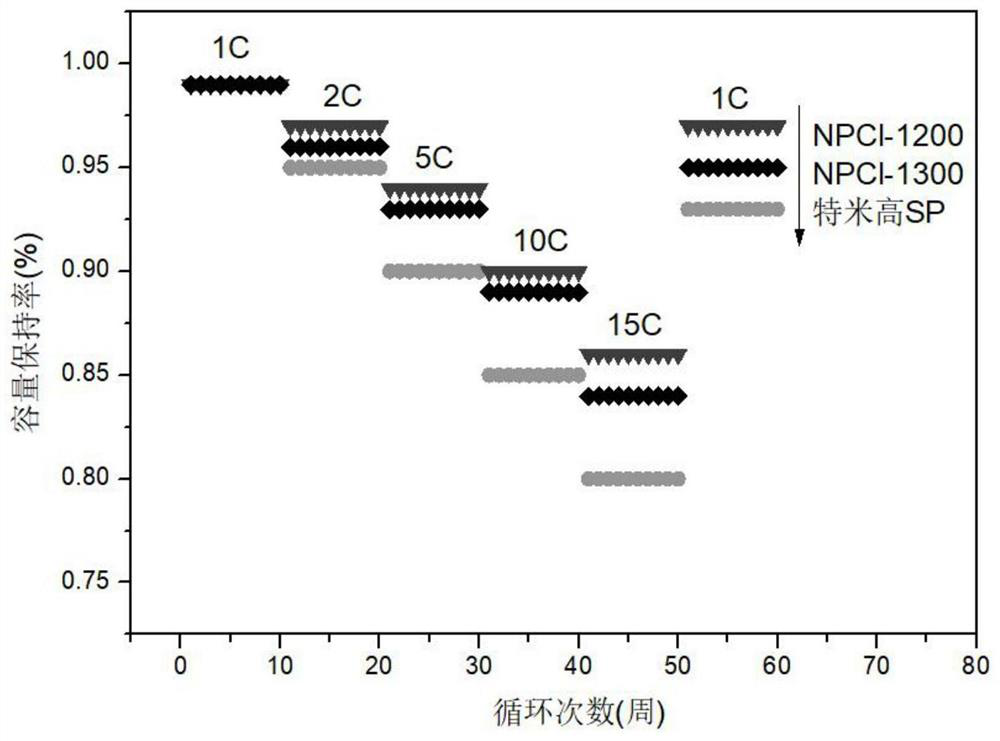
Nitrogen-phosphorus-chlorine co-doped carbon material, preparation method thereof and application of nitrogen-phosphorus-chlorine co-doped carbon material in lithium battery
A technology of co-doping and carbon materials, applied in the preparation/purification of carbon, battery electrodes, secondary batteries, etc., can solve the problem of limited energy density and cycle performance improvement of lithium-ion batteries, affecting the energy density of lithium-ion batteries, foreign elements Low doping amount and other issues, to achieve the effect of improving energy density and cycle performance, realizing scale and industrialization, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Phosphorus present invention is one kind of chlorine co-doped carbon material production method, comprising the steps of:
[0034] (1) precisely weighed 100g peanut shells washed, dried, pulverized and sieved through a 40 mesh screen to obtain spare peanut shell powder;
[0035] (2) respectively, said taking 50g of PCl 5 And 50g of NH 4 Kettle, the reaction was stirred for 4h at 120 deg.] C, to give a mixture of nitrogen and phosphorus-containing chlorine-Cl successively with 100g of methylene chloride was slowly added to a hydrothermal reaction;
[0036] (3) Step (1) Preparation of peanut shell powder was added in step (2) the mixture was stirred under nitrogen and phosphorus-containing chlorine obtained 7H room temperature, the stirred mixture was collected in a centrifuge tube, a rotational speed of 4000r / min centrifugal after 10 minutes, the supernatant liquid sucked, deionized water was added to the centrifuge tube, repeated five times, the supernatant was discarded ...
Embodiment 2
[0039] Phosphorus carbon material production method according to the present embodiment is chlorine co-doped with only in step (4) the difference in heat treatment temperature is different from the embodiment in Example 1, in this embodiment the heat treatment temperature of 800 deg.] C, N and P were prepared in chlorine referred to as a carbon material doped NPCL-800.
Embodiment 3
[0041] Distinction prepared carbon material of Example 1 of the present embodiment chloride Phosphorus co-doping embodiment only in that step (4) is different from the heat treatment temperature and time, in this embodiment the heat treatment temperature is 900 ℃, the heat treatment time 2h, nitrogen and phosphorus chloride of the co-doped material is referred to as a carbon NPCL-900.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com