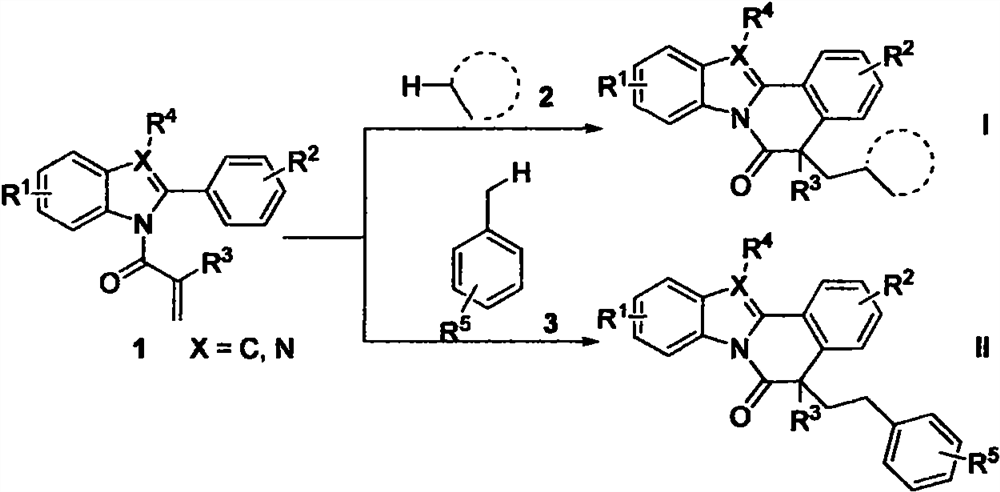
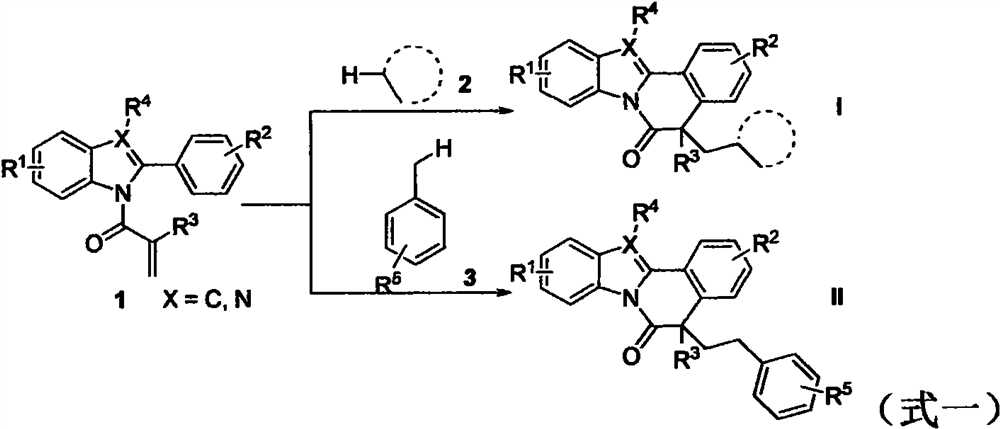
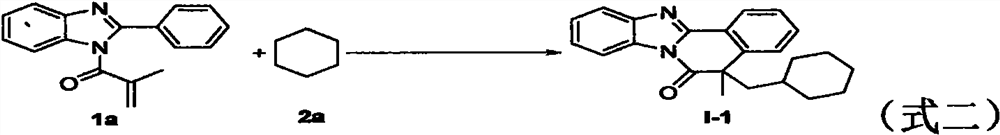
Free radical cyclization reaction method initiated by unactivated alkane C (sp3)-H functionalization
An unactivated and functionalized technology, applied in organic chemistry and other fields, can solve problems such as high bond dissociation energy and low polarity, and achieve a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0037] Embodiment 1-10 Reaction condition optimization experiment.
Embodiment 1
[0039]
[0040] In a Schlenk tube add 2-arylbenzimidazole 1a (52.4 mg, 0.2 mmol), cyclohexane 2a (0.2 mL), di-tert-butyl hydroperoxide (DTBP, 35.1 mg, 0.24 mmol), dodecane Sodium disulfate (SDS, 11.5mg, 0.04mmol) and H 2 O (1.8 mL). The reaction was then stirred at 90°C and sealed under air until complete consumption of starting material monitored by TLC or GC-MS analysis (reaction time 24 hours). After the reaction was completed, it was extracted three times with ethyl acetate. The organic layer was in Na 2 SO 4Dry on , solvent filtered and evaporated. The solution was concentrated under reduced pressure, and the mixture was purified by flash column chromatography to obtain the target product I-1 (86% yield); 1 H NMR (500MHz, CDCl 3 )δ: 8.50-8.49(m, 1H), 8.39-8.38(m, 1H), 7.84-7.83(m, 1H), 7.58-7.55(m, 1H), 7.49-7.41(m, 4H), 2.50- 2.46(m, 1H), 2.08-2.04(m, 1H), 1.66(s, 3H), 1.47-1.39(m, 3H), 1.27-1.24(m, 1H), 1.19-1.14(m, 1H), 1.00-0.88(m, 3H), 0.84-0.77(m, 3H); 1...
Embodiment 2
[0042] The oxidant uses tert-butyl peroxide (TBHP) instead of di-tert-butyl hydroperoxide (DTBP), and the rest of the conditions are the same as in Example 1, and the yield of the target product I-1 is 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com