Sulfonium salt-based stable HDAC-targeting polypeptide drug conjugate and application thereof
A conjugated and stable technology, applied in the field of bioengineering, can solve the problems of poor biosafety, multiple toxic and side effects, and achieve the effect of good biosafety, good biocompatibility, and increased therapeutic window.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Design of HDAC-targeted polypeptide drug conjugates
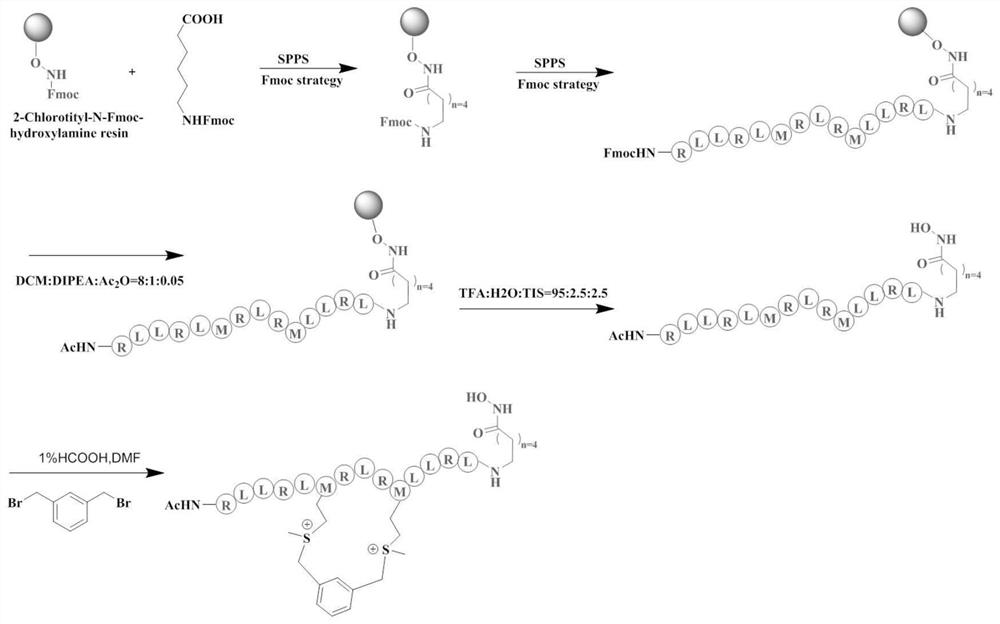
[0024] The present invention adopts the method of coupling HDAC inhibitors with sulfonium salt-stabilized apoptosis polypeptides to design HDAC-targeted polypeptide drug conjugates, such as figure 1 As shown, we selected the previously reported apoptotic polypeptide as the entry point (the original apoptotic polypeptide sequence: RLLRLLRLRRLRL, R is arginine, L is leucine), and the polypeptide was stabilized using the sulfonium salt-stabilized peptide methodology. Try to improve the stability of the peptide, and rely on the hydrophilicity of the sulfonium salt to change the hydrophilicity and hydrophobicity of the overall apoptosis peptide, and try to reduce the toxicity to normal cells. In order to further improve the anti-tumor activity of the polypeptide, we introduced the carbon-terminus of the polypeptide into the HDAC inhibitor hydroxamic acid structure, which has a broad spectrum of inhibitory activ...
Embodiment 2
[0026] Example 2: Synthesis and preparation of stable peptide drug conjugates targeting HDAC
[0027] (1) Resin swelling and deprotection:
[0028] The specific operation of solid-phase peptide synthesis is as follows: figure 2As shown in the roadmap: The special 2-Chlorotityl-N-Fmoc-hydroxylamine resin (loading degree: 0.54mmol / g) is used for the solid-phase synthesis of polypeptides with hydroxamic acid. The procedure was as follows: 2-Chlorotityl-N-Fmoc-hydroxylamine resin was swollen with DCM for 15 minutes. Use 50% morphine (dissolved in DMF) to remove the Fmoc protecting group for 30 minutes each time, twice in total, and then wash with DMF / DCM alternately for 3 times.
[0029] (2) Synthesis of resin polypeptide:
[0030] The above-mentioned deprotected resin, the prepared (1) Fmoc-6-amino-caproic acid (5eq, 0.4M, DMF) solution, 1H-benzotriazol-1-yloxytripyrrolidinyl hexafluorophosphate ( PyBOP) (5eq, 0.4M, DMF) solution and N,N-diisopropylethylamine (DIPEA) (10eq) ...
Embodiment 3
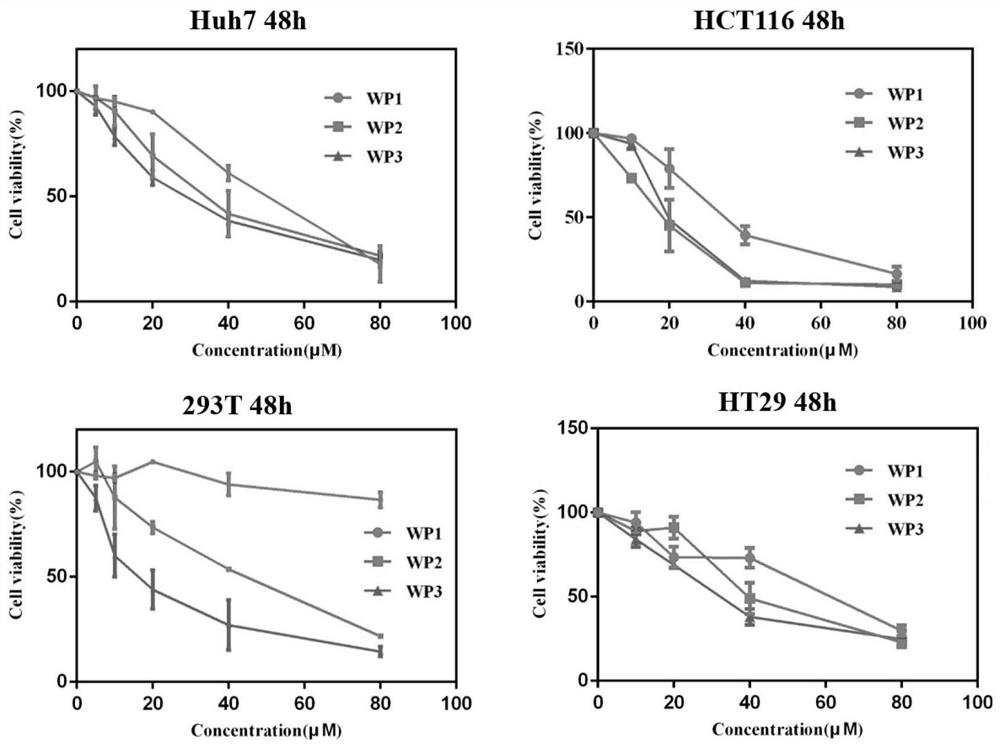
[0031] Example 3: Enzyme Activity Inhibition Effect of Stable Polypeptide Drug Conjugate Targeting HDAC
[0032] The identification of the inhibitory effect of HDAC enzyme activity was carried out with a commercially purchased HDAC enzyme activity detection kit, and each operation was repeated at least three times. HDAC enzymes came from HeLa nuclear extracts reported in many literatures. The enzyme activity results were based on commercial reagents. The operation of the box is carried out, and finally the inhibitory activity of the peptide drug conjugate on the HDAC protein is measured with a microplate reader, as shown in Table 1:
[0033] Table 1 Different polypeptide drugs designed by the present invention are to HDAC enzyme activity inhibitory effect figure
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com