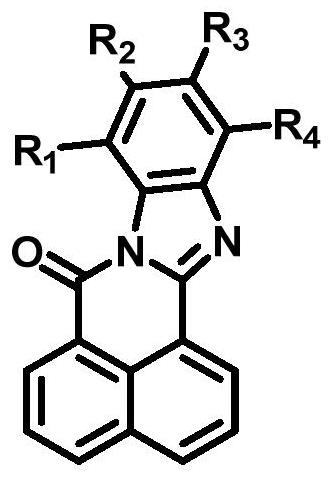
Application of broad-spectrum fluorescent probe for detecting cytochrome oxidase CYP3A
A cytochrome and fluorescent probe technology, applied in the field of two-photon fluorescent probes, can solve the problems of simultaneous detection of fluorescent probes, inability to effectively evaluate CYP3A inhibitory behavior, and inability to accurately reveal the inhibitory effect, and achieve the effect of sensitive detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1. the synthesis of compound BN-1
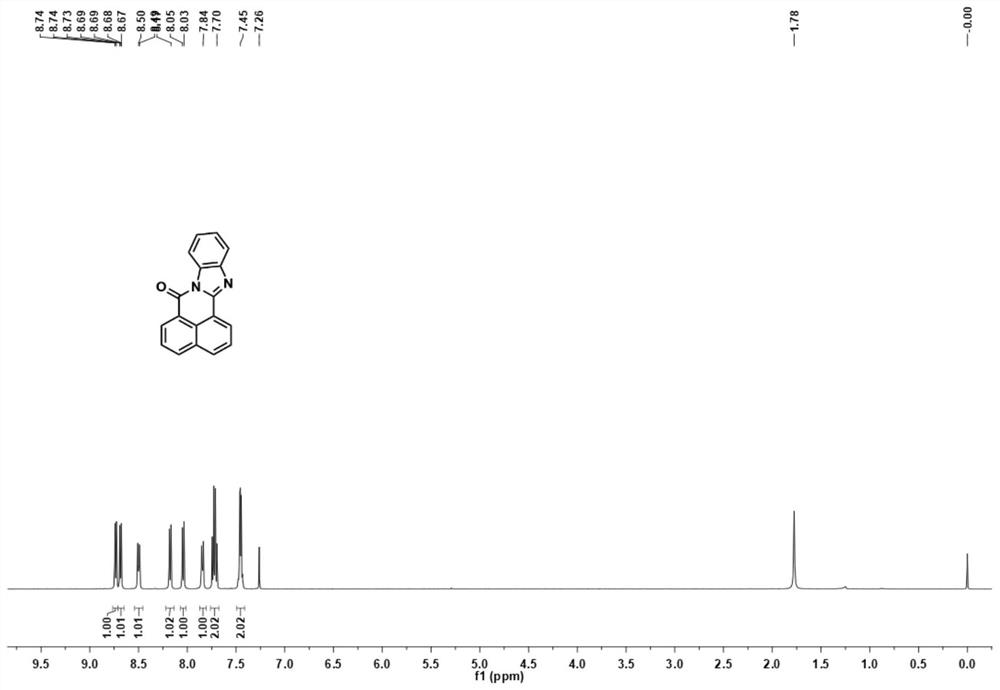
[0039] 1,8-Naphthalic anhydride (990.85 mg, 5.0 mmol) was dissolved in 20 mL of glacial acetic acid, o-phenylenediamine (648.84 mg, 6.0 mmol) was added, and the reaction solution was stirred and refluxed for 4 h. TLC traced the end of the reaction, and the reaction solution was allowed to stand and cooled to room temperature, and a large amount of solids were precipitated, filtered, and the filter cake was washed with ethanol to obtain a crude product, which was further separated by silica gel column (developing agent was dichloromethane) to obtain compound BN-1 (1272.07 mg, yield 94.2%) as a yellow-green solid.
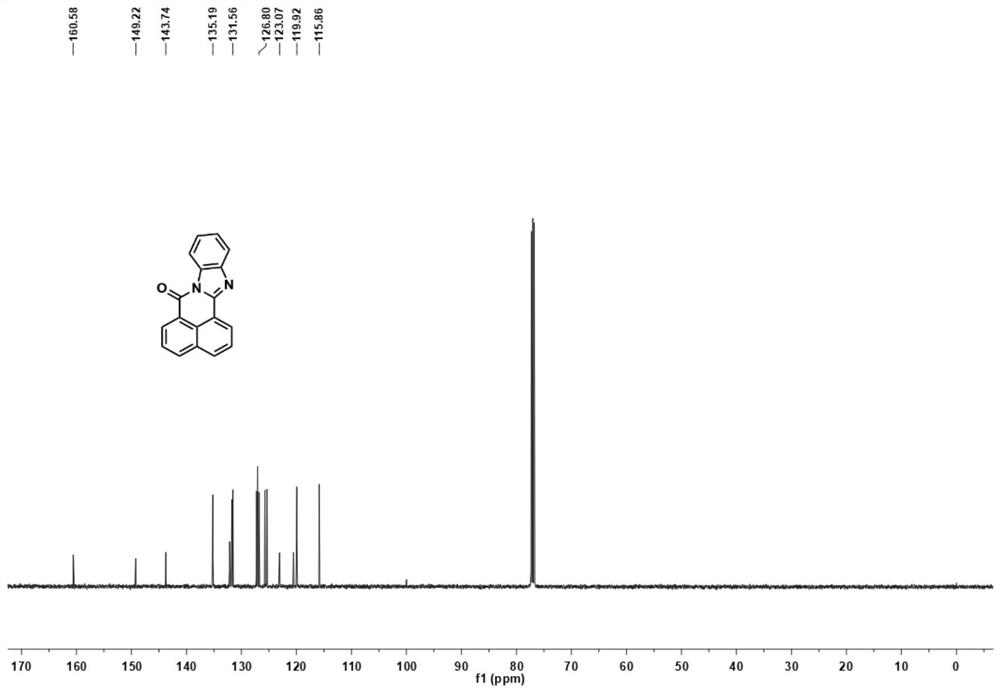
[0040] BN-1: 1 H NMR (500MHz, CDCl 3 )δ8.76–8.71(m,1H),8.68(dd,J=7.3,0.9Hz,1H),8.54–8.45(m,1H),8.17(d,J=8.1Hz,1H),8.04(d ,J=8.1Hz,1H),7.87–7.81(m,1H),7.72(dd,J=15.9,8.3Hz,2H),7.49–7.41(m,2H). 13 C NMR (125MHz, CDCl 3 )δ160.58, 149.22, 143.74, 135.19, 132.15, 131.82, 131.75, 131.56, 127.27, 127.06, 126.80, 125.7...
Embodiment 3
[0042] Embodiment 3. In vitro determination of the selectivity of human recombinant CYP single enzyme
[0043] Prepare 180 μL CYP metabolic reaction system in advance, including Buffer buffer (100mM) at pH 7.4, glucose-6-phosphate (10mM), glucose-6-phosphate dehydrogenase (1unit / mL), MgCl 2 (4mM), recombinant human CYP single enzyme, the final concentration of BN-1 is 10μM, pre-incubated with shaking at 37°C for 3 minutes; add 20μL of 10mM NADP to the reaction system + Initiate the reaction; after 60 minutes, add 100 μL of glacial acetonitrile, shake vigorously, and terminate the reaction; use a high-speed refrigerated centrifuge to centrifuge at 20,000×g for 20 minutes at 4°C, take the supernatant, and perform fluorescence detection ( E. x =470nm,E m = 526nm).
[0044] From Figure 5 It can be seen that the probe has good selectivity to recombinant human CYP3A4 and CYP3A5 enzymes, and the fluorescence response to CYP3A4 and CYP3A5 is close, while other enzymes of the CYP ...
Embodiment 4
[0045] Example 4. Determination of CYP3A protein concentration standard curve
[0046] The experiment was carried out on a microplate reader using a 96-well plate, 10 μM BN-1, 6-phosphate glucose (10 mM), glucose-6-phosphate dehydrogenase (1 unit / mL), MgCl 2 (4mM)NADP + (1mM), CYP3A4 and CYP3A5 single enzyme (0-15nM), pH 7.4 Buffer buffer 100mM, total volume 200μL, incubated at 37℃ for 30min, the ratio of the fluorescence intensity of the product to the fluorescence intensity of the substrate and the protein concentration standard curve line.
[0047] Figure 6 Middle a) is the fluorescence intensity curve catalyzed by different concentrations of CYP3A4, b) is the linear relationship between CYP3A4 protein concentration and fluorescence intensity; c) is the fluorescence intensity curve catalyzed by different concentrations of CYP3A5, d) is the linear relationship between CYP3A5 protein concentration and fluorescence intensity . The results indicated that the probe substrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com