Method for detecting genetic toxic impurities of Adenafil citrate
A genotoxic and citric acid technology, which is applied in the field of detection of genotoxic impurities of idenafil citrate, can solve the problems of inability to control quality, low sensitivity, etc., and achieve detection sensitivity and accuracy improvement, detection Improved sensitivity and high reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
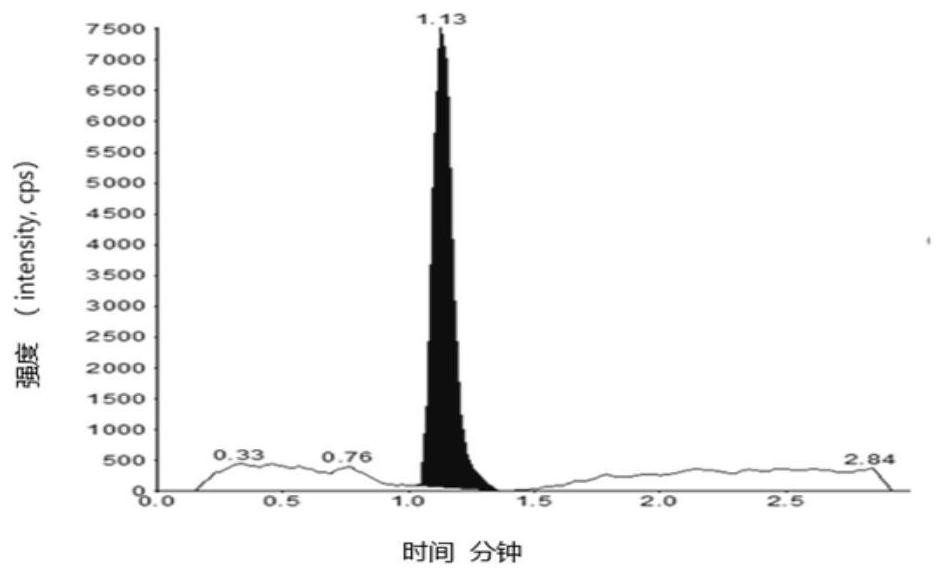
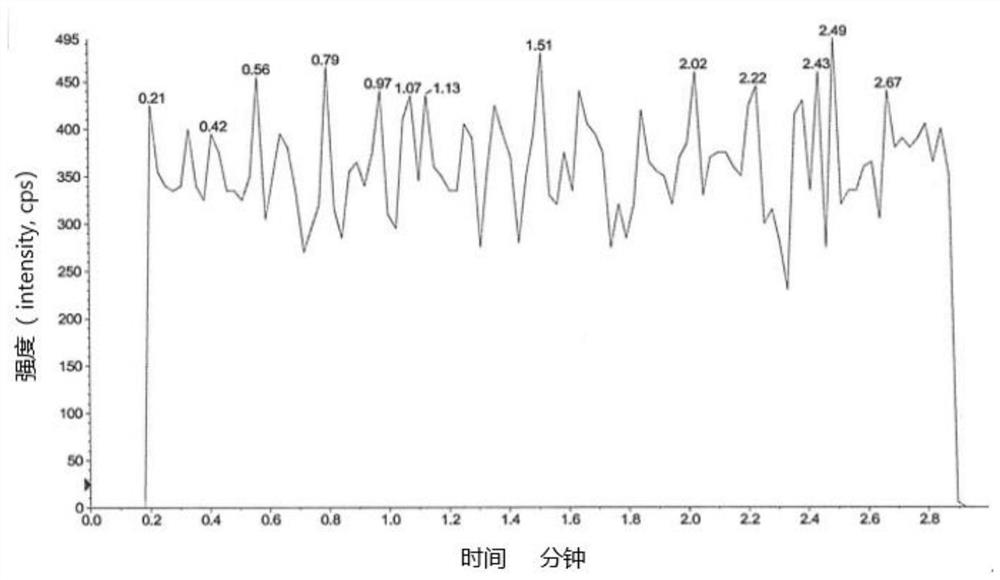
[0093] Embodiment 1 specificity test
[0094] Blank solvent: 60% aqueous methanol;
[0095] Preparation of the test sample: take an appropriate amount of edenafil, weigh it accurately, dissolve it with dimethyl sulfoxide to a 0.1g / mL stock solution, and then dilute it with a diluent to a 2mg / mL solution;
[0096] Preparation of control solution: Take an appropriate amount of impurity A reference substance, dissolve it in methanol to make a 1mg / mL reference substance stock solution, and then dilute it to make 100μg / mL reference substance stock solution 1 and 10μg / mL reference substance stock solution 2.
[0097] Preparation of control solution: Take reference substance stock solution 1 and reference substance stock solution 2 to make a 10 μg / mL reference substance solution. Then take the reference substance solution and the 0.1g / mL test solution and dilute with the diluent to make a 1.00ng / mL solution respectively.
[0098] Preparation of system suitability solution: Take ref...
Embodiment 2
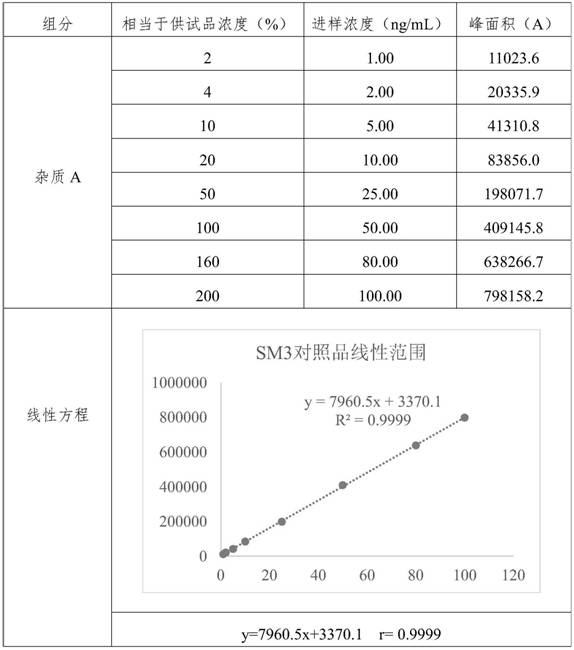
[0105] Embodiment 2 linearity and range test
[0106] Diluent: 60% methanol water solution.
[0107] Preparation of the test sample: take an appropriate amount of edenafil, accurately weigh it, and dissolve it with dimethyl sulfoxide to form a 0.1g / mL stock solution;
[0108] Preparation of control solution: Take an appropriate amount of impurity A reference substance, dissolve it in methanol to make a 1mg / mL reference substance stock solution, and then dilute it to make 100μg / mL reference substance stock solution 1 and 10μg / mL reference substance stock solution 2.
[0109] Preparation of linear solution of reference substance: Take reference substance stock solution 1 and reference substance stock solution 2 to make 1μg / mL, 2μg / mL, 5μg / mL, 10μg / mL, 25μg / mL, 50μg / mL, 80μg / mL, 100μg / mL and a series of linear reference solutions. Then take a series of linear reference solution and 0.1g / mL test solution and dilute with diluent to make 1ng / mL, 2ng / mL, 5ng / mL, 10ng / mL, 25ng / mL, ...
Embodiment 3
[0114] Embodiment 3 detection limit, quantitative limit
[0115] Take an appropriate amount of the impurity A reference substance, prepare it into a reference substance solution, and prepare it into a test solution through step-by-step dilution. When the three-fold noise value S / N ≥ 3, it is used as the detection limit; when S / N ≥ 10, as the limit of quantitation. The results are shown in the table below.
[0116] Table 4 Detection limit results
[0117]
[0118] Table 5 Quantitation limit results
[0119]
[0120] Conclusion: The detection limit of genotoxic impurities in this product is 0.40ng / mL, which is equivalent to 0.2ppm of the test solution; the quantification limit is 1.00ng / mL, which is equivalent to 0.5ppm of the test solution. The precision RSD of 6-needle injection for quantitative concentration determination was 5.40%. It meets the sensitivity requirements for the determination of genotoxic impurities of this product.
[0121] Embodiment 3 recovery te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com