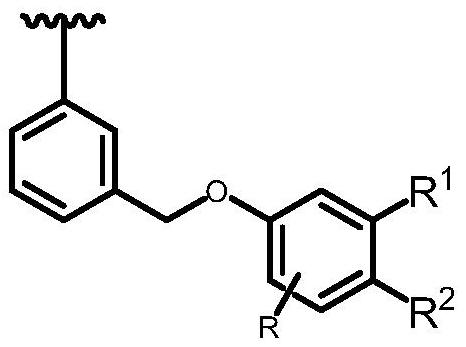
Chelate resin as well as preparation method and application thereof
A technology of chelating resins and resins, which is applied in the field of chelating resins and its preparation, can solve the problems of slow adsorption speed, poor hydrophilicity, and small adsorption capacity, and achieve good product quality, high elution rate, and high selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] A kind of preparation method (R group is methyl) of 2-hydroxy-6-methylbenzoic acid chelate resin, comprises the following steps:
[0100] (1) Pretreatment: In a 250mL beaker, add 30g of chlorine balls (4% cross-linking degree), 100mL of deionized water, stir at room temperature, repeat the above washing 3 times, then suction filter, then add 100mL of absolute ethanol , stirred, and repeated washing 3 times. Suction filtration to obtain clean chlorine balls for later use. (2) Esterification reaction: In a 200 mL three-necked flask, 12.00 g of 2,4-dihydroxy-6-methylbenzoic acid, 60 mL of methanol, and 5 mL of sulfuric acid were added, and the mixture was refluxed for 20 h. After the reaction was completed, it was cooled to room temperature, methanol was removed under reduced pressure, and the residue was poured into 200 mL of ice water. The precipitate was collected and washed with water. The solid was recrystallized (methanol / hexanes) to give the product methyl 2,4-di...
Embodiment 2
[0102] A kind of preparation method of 2-hydroxymethylbenzoic acid chelating resin (R 1 base is CH 2 OH), including the following steps:
[0103](1) Pretreatment: In a 250mL beaker, add 30g of chlorine balls (4% cross-linking degree), 100mL of deionized water, stir at room temperature, repeat the above washing 3 times, then suction filter, then add 100mL of absolute ethanol , stirred, and repeated washing 3 times. Suction filtration to obtain clean chlorine balls for later use. (2) Esterification reaction: In a 200 mL three-necked flask, 12.00 g of 2-hydroxymethyl-4-hydroxybenzoic acid, 60 mL of methanol, and 5 mL of sulfuric acid were added, and the mixture was refluxed for 20 h. After the reaction, it was cooled to room temperature, methanol was removed under reduced pressure, and the residue was poured into 200 mL of ice water. The precipitate was collected and washed with water. The solid was recrystallized (methanol / hexanes) to give the product methyl 2-hydroxymethyl...
Embodiment 3
[0105] A kind of preparation method of o-hydroxyphenylacetic acid chelating resin (R 2 base is CH 2 COOH), including the following steps:
[0106] (1) Pretreatment: In a 250mL beaker, add 30g of chlorine balls (4% cross-linking degree), 100mL of deionized water, stir at room temperature, repeat the above washing 3 times, then suction filter, then add 100mL of absolute ethanol , stirred, and repeated washing 3 times. Suction filtration to obtain clean chlorine balls for later use. (2) Esterification reaction: In a 200 mL three-necked flask, 12.00 g of 2,4-dihydroxyphenylacetic acid, 60 mL of methanol, and 5 mL of sulfuric acid were added, and the mixture was refluxed for 20 h. After the reaction, cooled to room temperature, methanol was removed under reduced pressure, and the residue was poured into 200 mL of ice water. The precipitate was collected and washed with water. The solid was recrystallized (methanol / hexanes) to give the product methyl 2,4-dihydroxyacetate as a w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com