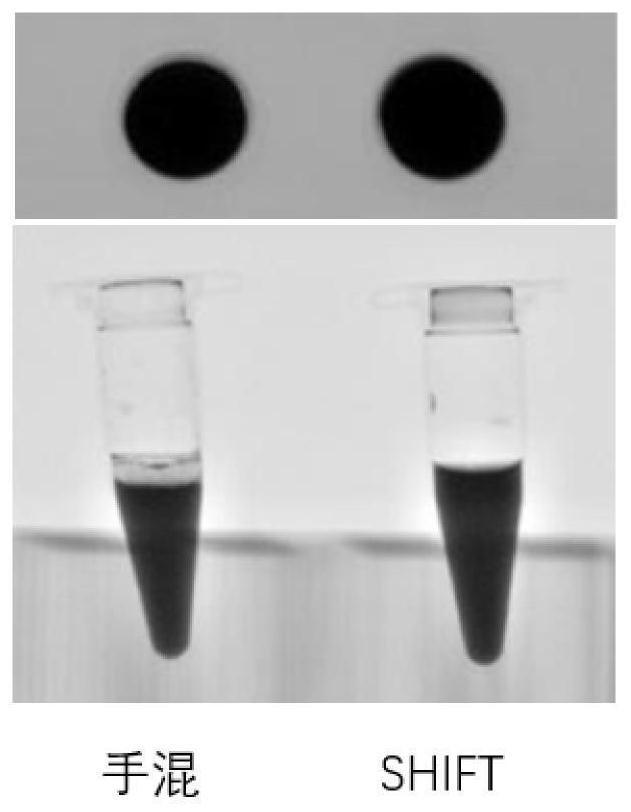
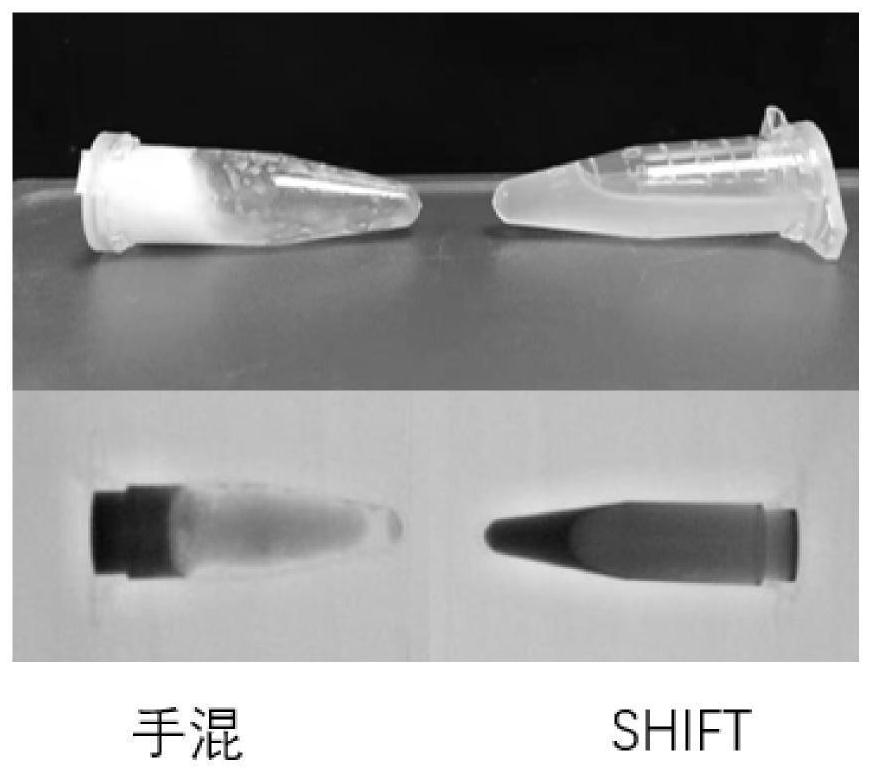
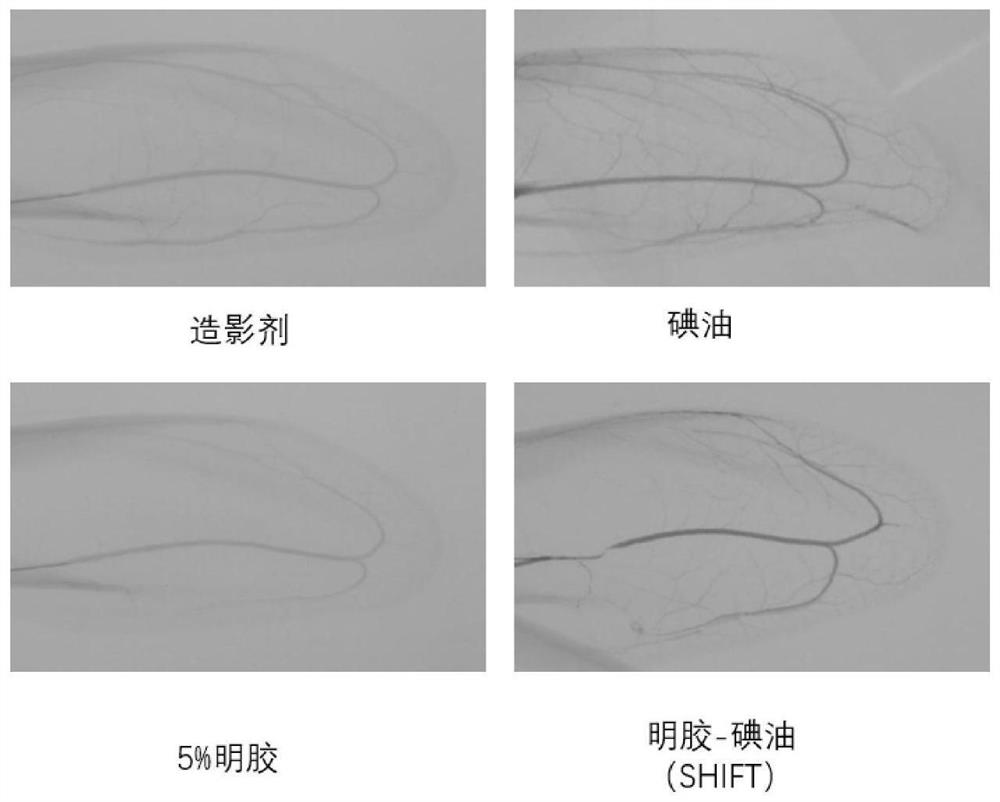
Preparation method and application of injectable gelatin-iodized oil homogeneous preparation for vascular embolism
A technology of vascular embolism and gelatin, which is applied in the field of preparation of injectable gelatin-iodol homogeneous preparations for vascular embolism, can solve the problems of inability to improve the loss and loss of lipiodol, incomplete tumor embolization, tumor recurrence, etc., and achieve embolism-enhancing drugs carrying effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) The medical gelatin sponge particles are mixed with sterile water, then swelled in a water bath at 50° C. to form liquid gelatin, wherein the concentration of gelatin in the sterile water is 20 wt %;
[0035] (2) The liquid gelatin prepared in step (1) and the lipiodol injection containing 48.0% iodine are added to the autoclave at a volume ratio of 0.1:1, pressurized to 20MPa, and the temperature is controlled at 50°C. After the temperature and pressure are stabilized, keep the temperature and pressure and stir for 1 hour;
[0036] (3) After decompression, the prepared injectable gelatin-iodol homogeneous preparation for vascular embolism was collected from the autoclave.
Embodiment 2
[0038] (1) Medical gelatin sponge particles are mixed with DOX-dissolved sterile water, then swollen with a 50°C water bath to form liquid gelatin, wherein the concentration of gelatin in sterile water is 20wt%, and the concentration of DOX in sterile water is 10mg / mL;
[0039] (2) The liquid gelatin prepared in step (1) and the lipiodol injection containing iodine of 48.0% are added to the autoclave at a volume ratio of 0.1:1, pressurized to 20MPa, and the temperature is controlled at 40°C. After the temperature and pressure are stabilized, keep the temperature and pressure and stir for 1 hour;
[0040] (3) After decompression, the prepared hepatic arterial chemoembolization agent was collected from the autoclave.
Embodiment 3
[0042] (1) the medical gelatin sponge particle is mixed with the aseptic water that dissolves water-soluble drug rhodamine, then it is swollen to form liquid gelatin with 50 ℃ of water baths, and wherein the concentration of gelatin in sterile water is 20wt%, rhodamine is in the absence of The concentration in bacterial water is 1mg / mL;
[0043] (2) The liquid gelatin prepared in step (1) and the lipiodol injection containing iodine of 48.0% are added to the autoclave at a volume ratio of 0.1:1, pressurized to 20MPa, and the temperature is controlled at 40°C. After the temperature and pressure are stabilized, keep the temperature and pressure and stir for 1 hour;
[0044] (3) After decompression, collect the prepared hepatic arterial chemoembolization agent from the autoclave;
[0045] (4) Dispersing the fat-soluble drug coumarin-6 in step (3) to prepare a composite embolic agent, wherein the concentration of coumarin-6 in the composite embolic agent is 1 mg / mL.
[0046] Com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com