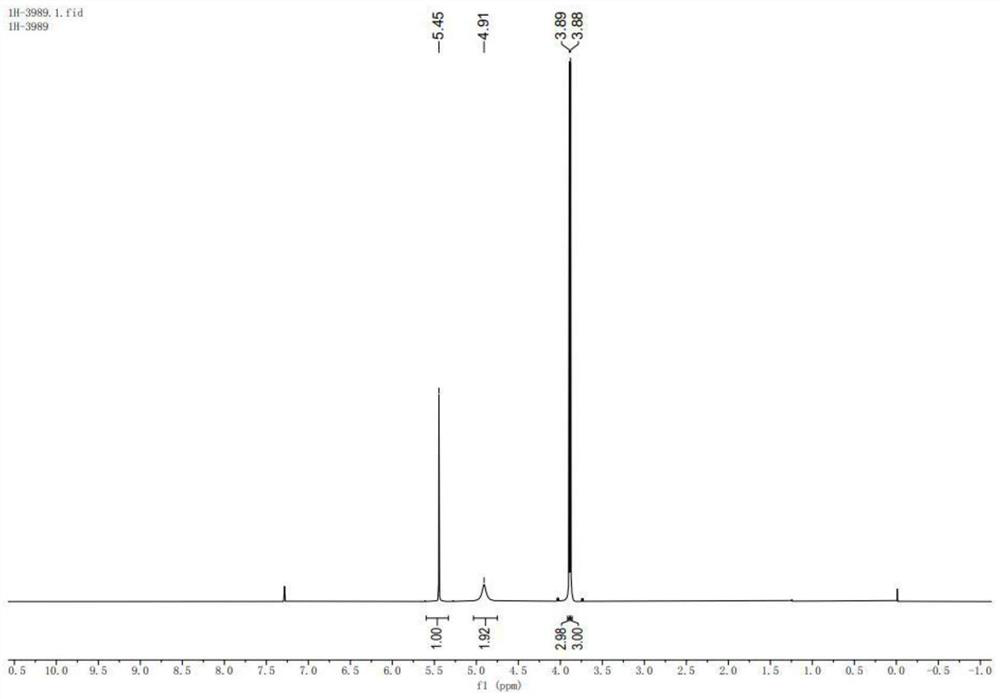
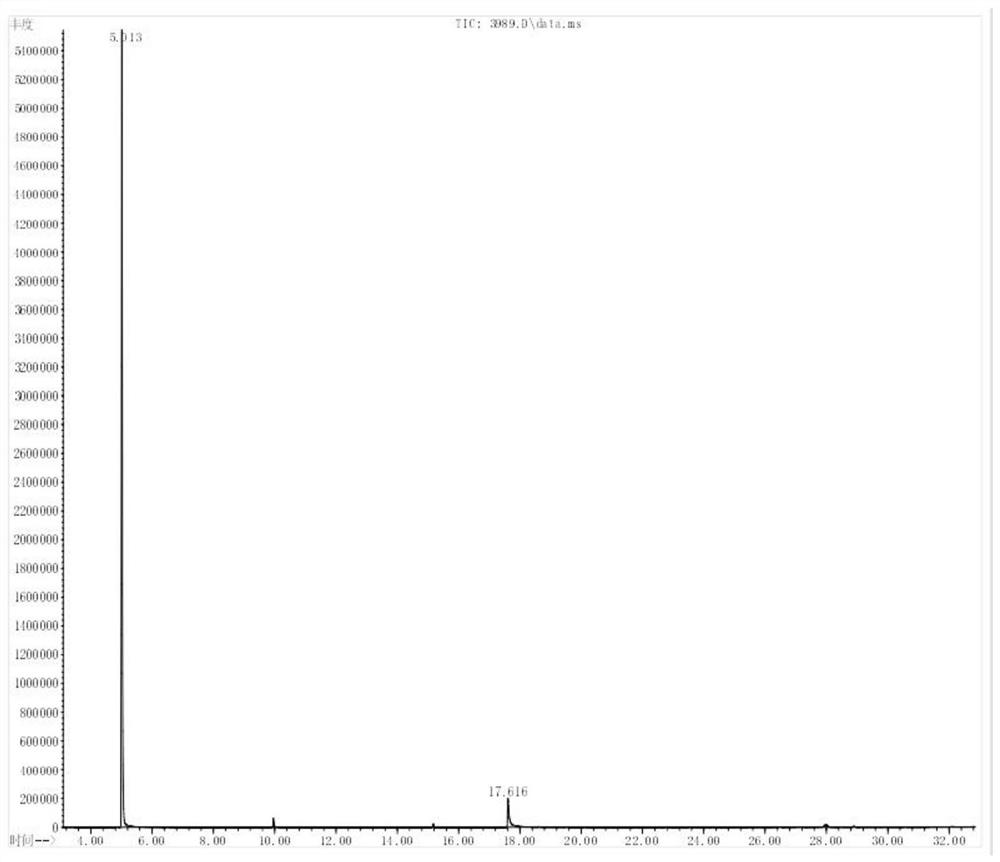
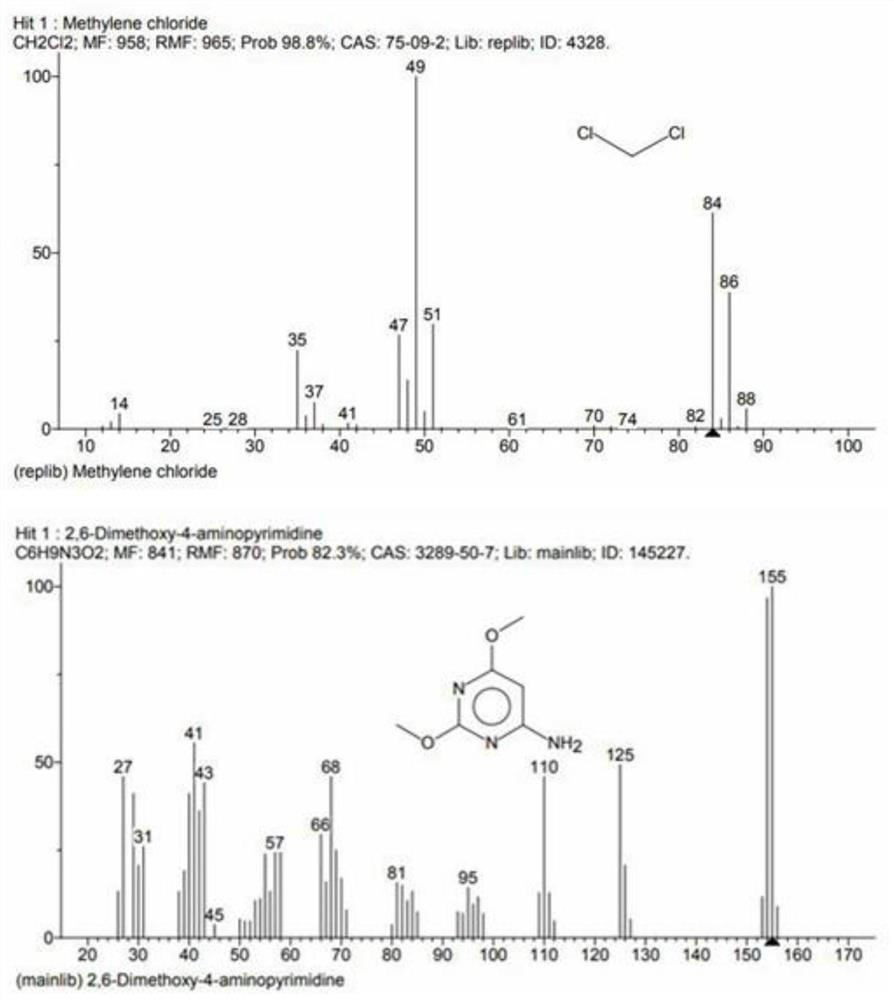
Preparation method of environment-friendly 4-amino-2, 6-dimethoxy pyrimidine
An environmentally friendly technology of dimethoxypyrimidine, which is applied in organic chemistry and other fields, can solve the problems of long reaction steps, safety risks, and high costs, and achieve the effects of fewer reaction steps, high safety, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A method for preparing environmentally friendly 4-amino-2,6-dimethoxypyrimidine, comprising the steps of:
[0044] (1) Add 49 g of 2-methoxyformamidine and 66 g of methyl cyanoacetate into a 500 mL four-necked reaction flask, stir evenly, heat to 80° C., and perform cyclization reaction for 6 h. After the reaction was completed, it was cooled to room temperature, a solid was precipitated, and filtered to obtain 80.8 g of 4-amino-2-methoxy-6-hydroxypyrimidine with a yield of 86%.
[0045] (2) Add 80.8 g of 4-amino-2-methoxy-6-hydroxypyrimidine obtained in step (1) into a 1L three-necked flask containing 323.2 g of phosphorus oxychloride, heat to 90°C, and chlorinate After 4 hours, after recovering excess phosphorus oxychloride under reduced pressure, the remaining product was slowly added to ice water, adjusted to pH 8 with sodium bicarbonate solution with a pH value of 4, and the precipitated solid was filtered and dried to obtain 4- Amino-2-methoxy-6-chloropyrimidine ...
Embodiment 2
[0049] A method for preparing environmentally friendly 4-amino-2,6-dimethoxypyrimidine, comprising the steps of:
[0050] (1) Add 49 g of 2-methoxyformamidine and 66 g of methyl cyanoacetate into a 500 mL four-necked reaction flask, stir evenly, heat to 80° C., and perform cyclization reaction for 6 h. After the reaction was completed, it was cooled to room temperature, and a solid was precipitated. After filtration, 78.9 g of 4-amino-2-methoxy-6-hydroxypyrimidine was obtained, with a yield of 84%.
[0051] (2) Add 78.9 g of 4-amino-2-methoxy-6-hydroxypyrimidine obtained in step (1) into a 1L three-necked flask containing 315.8 g of phosphorus oxychloride, heat to 90°C, and chlorinate After 4 hours, after the excess phosphorus oxychloride was recovered under reduced pressure, the remaining product was slowly added to ice water, adjusted to a pH of 8 with a sodium bicarbonate solution with a pH value of 5, and the precipitated solid was filtered and dried to obtain 4- Amino-2-...
Embodiment 3
[0055] A method for preparing environmentally friendly 4-amino-2,6-dimethoxypyrimidine, comprising the steps of:
[0056] (1) Add 49 g of 2-methoxyformamidine and 66 g of methyl cyanoacetate into a 500 mL four-necked reaction flask, stir evenly, heat to 60° C., and carry out cyclization reaction for 6 h. After the reaction was completed, it was cooled to room temperature, and a solid was precipitated. After filtration, 4-amino-2-methoxy-6-hydroxypyrimidine was obtained with a yield of 63%.
[0057] (2) Add 59.2 g of 4-amino-2-methoxy-6-hydroxypyrimidine obtained in step (1) into a 1 L three-necked flask containing 265 g of phosphorus oxychloride, heat to 90° C., and chlorinate for 4 h Finally, after the excess phosphorus oxychloride was recovered under reduced pressure, the remaining product was slowly added to ice water, adjusted to a pH of 10 with a sodium bicarbonate solution with a pH value of 4, and the precipitated solid was filtered and dried to obtain 4-amino -54.7 g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com