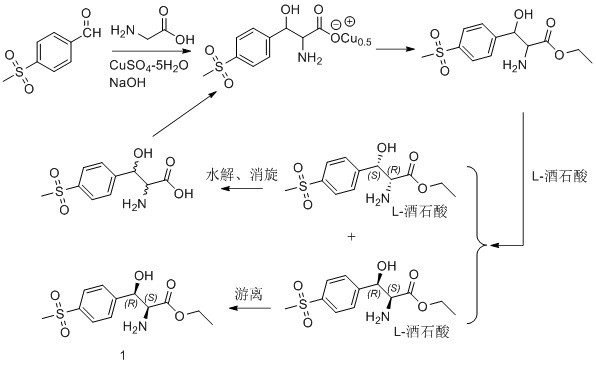
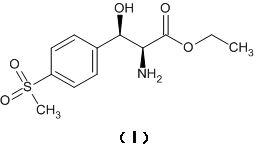
Asymmetric synthesis method for preparing (2S, 3R)-p-methylsulfonyl phenyl serine ethyl ester
A technology of methylsulfonyl phenylserine ethyl ester and synthesis method, which is applied in the field of asymmetric synthesis of preparation-p-methylsulfonyl phenylserine ethyl ester, can solve the problems of complex process, low yield, many steps and the like, and achieves the process route The effect of brief, high product yield, and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] Such as figure 2 As shown, during specific implementation, the asymmetric synthesis method for preparing (2S, 3R)-p-thymphenylphenylserine ethyl ester of the present invention comprises the following steps:
[0031] The first step is to asymmetrically reduce the ketone carbonyl of the latent chiral ketone carbonyl compound with a chiral reagent, and the obtained reaction product is (3R)-p-thymphenylphenylserine ethyl ester; the chiral reagent is (-)-diiso Pine camphenyl boron chloride, the latent chiral ketone carbonyl compound is ethyl 2-amino-3-[4-(methylsulfonyl)phenyl]-3-oxopropionate; wherein, (-)- The molar ratio of diisopine camphenyl boron chloride to ethyl 2-amino-3-[4-(thymphenyl)phenyl]-3-oxopropionate is 1.1~3:1. Note: The structural formula of (-)-diisopine camphenyl boron chloride is as follows:
[0032]
[0033] The specific process is: under the protection of an inert gas, dissolve (-)-diisopine camphenyl boron chloride in an appropriate amount of ...
Embodiment 1
[0039] A. Synthesis of (3R)-p-thymphenylphenylserine ethyl ester
[0040] Under nitrogen protection, the (Ipc) 2 Add 6.4g (20mmol) of BCl and 20ml of anhydrous THF into the reaction flask, and cool to 0°C. Dissolve 3.8g (13.3mmol) of ethyl 2-amino-3-[4-(methylsulfonyl)phenyl]-3-oxopropanoate in 10ml of anhydrous THF, slowly drop into the reaction flask, and control the temperature At 0°C~5°C. Stir for 24 hours. Slowly add ice water, stir for half an hour and then rise to room temperature, and continue stirring for 1 hour. Dichloromethane was added for extraction, and the organic phase was spin-dried to obtain a light yellow solid, namely (3R)-ethyl p-thymphenylphenylserine, with a yield of 82%.
[0041] B. Synthesis of (2S,3R)-p-thymphenylphenylserine ethyl ester
[0042] Add 5.75g (20mmol) of compound (3R)-p-thymphenylphenylserine ethyl ester and 20g of ethanol into the reaction flask, add 0.17g (1mmol) of 5-nitrosalicylaldehyde as a catalyst, and heat to 60°C for 30 Mi...
Embodiment 2
[0044] A. Synthesis of (3R)-p-thymphenylphenylserine ethyl ester
[0045] Under nitrogen protection, the (Ipc) 2 Add 9.6g (30mmol) of BCl and 20ml of anhydrous THF into the reaction flask, and cool to 0°C. Dissolve 3.8g (13.3mmol) of ethyl 2-amino-3-[4-(methylsulfonyl)phenyl]-3-oxopropanoate in 10ml of anhydrous THF, slowly drop into the reaction flask, and control the temperature At 0°C~5°C. Stir for 24 hours. Slowly add ice water, stir for half an hour and then rise to room temperature, and continue stirring for 2 hours. Dichloromethane was added for extraction, and the organic phase was spin-dried to obtain a light yellow solid, namely (3R)-ethyl p-thymphenylphenylserine, with a yield of 84%.
[0046] B. Synthesis of (2S,3R)-p-thymphenylphenylserine ethyl ester
[0047] Add 5.75g (20mmol) of compound (3R)-p-thymphenylphenylserine ethyl ester and 30g of ethanol to the reaction flask, add 0.12g (0.72mmol) of 5-nitrosalicylaldehyde as a catalyst, and heat to reflux Reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com