Cyclic phosphamide derivative with biaryl skeleton and synthesis method and application thereof
A cyclic phosphoramide and a synthesis method technology, applied in the field of synthesis, cyclic phosphoramide derivatives, can solve the problems of increasing economic cost, waste of resources and manpower, harsh reaction conditions, etc., to avoid waste, less pollution, and reaction cycle. short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
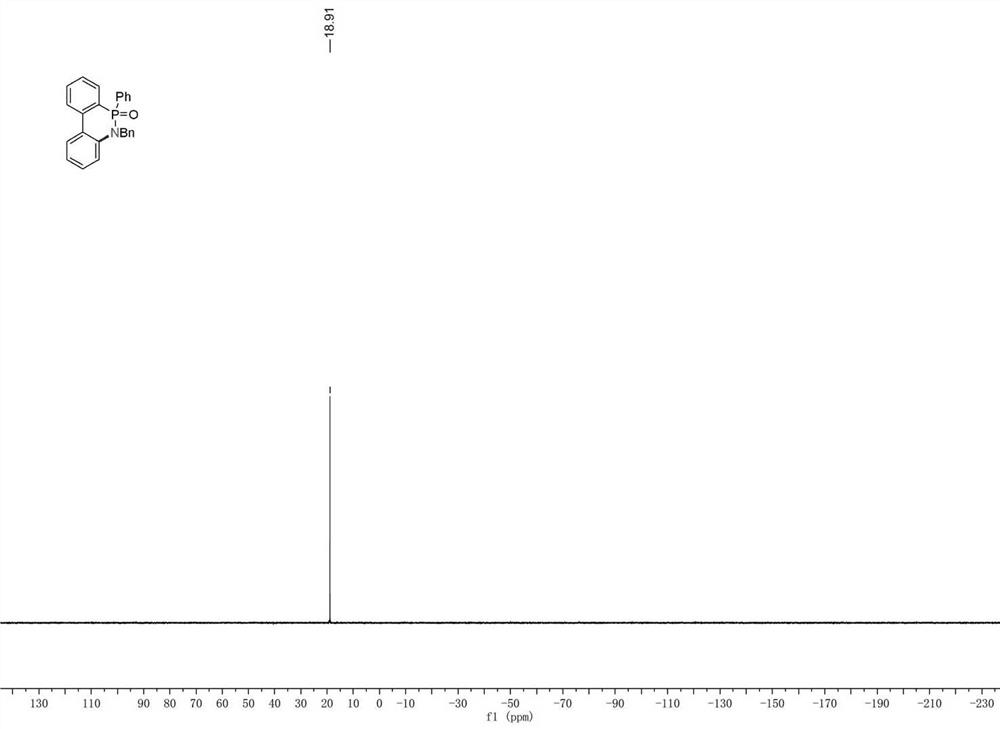
[0027] A kind of synthetic method of 6-phenyl-5 benzyl-5H-dibenzo[c,e][1,2]-6-oxaphosphonamide (2a), comprising the following steps: adding Phosphoramide 0.2mmol (77mg), n-butylammonium bromide (0.5mmol), 5mL acetonitrile / methanol mixed solution, put graphite electrodes and platinum electrodes into the reaction device, connect the power supply and feed 10mA constant current, and react at room temperature After 6 hours, it was concentrated under reduced pressure and subjected to column chromatography to obtain 72 mg of a white solid with a yield of 93% and a purity of 99.9%. 1 H NMR (400MHz, CDCl 3)δ8.20–7.97(m,2H),7.84-7.75(m,1H),7.74-7.60(m,3H),7.51-7.40(m,2H),7.39–7.30(m,2H),7.31– 7.11(m,6H),7.10–7.03(m,1H),7.02–6.96(m,1H),5.18(dd,J=16.8,6.8Hz,1H),4.72(dd,J=16.8,7.5Hz, 1H); 13 C{ 1 H}NMR (100MHz, CDCl 3 )δ139.7, 137.1 (d, J = 5.5Hz), 136.7 (d, J = 3.3Hz), 132.5 (d, J = 2.1Hz), 132.1 (d, J = 2.4Hz), 131.7, 131.6, 131.0 (d ,J=10.3Hz),129.8,128.6,128.5,128.5,127.6,127.5,12...
Embodiment 2
[0030] A method for synthesizing 4-methyl-6-phenyl-5-benzyl-5H-dibenzo[c,e][1,2]-6-oxaphosphonamide (2b), comprising the following steps:
[0031] Add phosphoramide 0.2mmol (81mg), n-butylammonium bromide (0.5mmol), 5mL acetonitrile / methanol mixed solution into the reaction vessel at one time, insert graphite electrode and platinum sheet electrode into the reaction device, connect the power supply and feed 10mA constant current, reaction at room temperature for 6h, concentration under reduced pressure, and column chromatography (200-300 mesh silica gel, petroleum ether / ethyl acetate = 2 / 1-5 / 1) to obtain 74 mg of white solid with a yield of 91%. 99.9% pure. 1 H NMR (400MHz, CDCl 3 )δ8.00–7.89(m,1H),7.88-7.78(m,1H),7.77–7.65(m,1H),7.65–7.47(m,3H),7.40–7.22(m,4H),7.23– 7.16(m,2H),7.13–7.03(m,3H),6.87–6.59(m,2H),5.09(dd,J=16.7,7.2Hz,1H),4.62(dd,J=16.8,7.4Hz, 1H), 2.14(s,3H); 13 C NMR (100MHz, CDCl 3 )δ156.31, 155.08, 142.44, 131.70, 130.23, 129.71, 128.25, 127.86, 127.27, 126...
Embodiment 3
[0034] A method for synthesizing 4,6-diphenyl-5-benzyl-5H-dibenzo[c,e][1,2]-6-oxaphosphonamide (2c), comprising the following steps:
[0035] Add phosphoramide 0.2mmol (78mg), n-butylammonium bromide (0.5mmol), 5mL acetonitrile / methanol mixed solution into the reaction vessel at one time, insert graphite electrode and platinum sheet electrode into the reaction device, connect the power supply and feed 10mA constant current, reaction at room temperature for 6h, concentration under reduced pressure, and column chromatography (200-300 mesh silica gel, petroleum ether / ethyl acetate = 2 / 1-5 / 1) to obtain 65 mg of white solid, yield 83%, purity 99.9%. White solid in 83% yield; 1 H NMR (400MHz, CDCl 3 )δ8.08–7.90(m, 2H),7.79–7.69(m,1H),7.68–7.55(m,3H),7.43–7.33(m,2H),7.33–7.20(m,10H),7.19– 7.15(m,3H),7.14–7.01(m,1H),5.17(dd,J=16.6,6.7Hz,1H),4.67(dd,J=16.6,7.1Hz,1H); 13 C NMR (101MHz, CDCl 3 )δ142.35, 140.31, 139.90(d, J=0.8Hz), 136.86, 132.51(d, J=2.0Hz), 132.14(d, J=2.7Hz), 131....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com