Key exchange domain for controlling lipid chain length change of lipopeptide, mutants and application of mutants
A technology for exchanging structures and domains, applied in the biological field, to achieve the effects of reducing toxic side effects, promoting clinical drug production, and increasing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
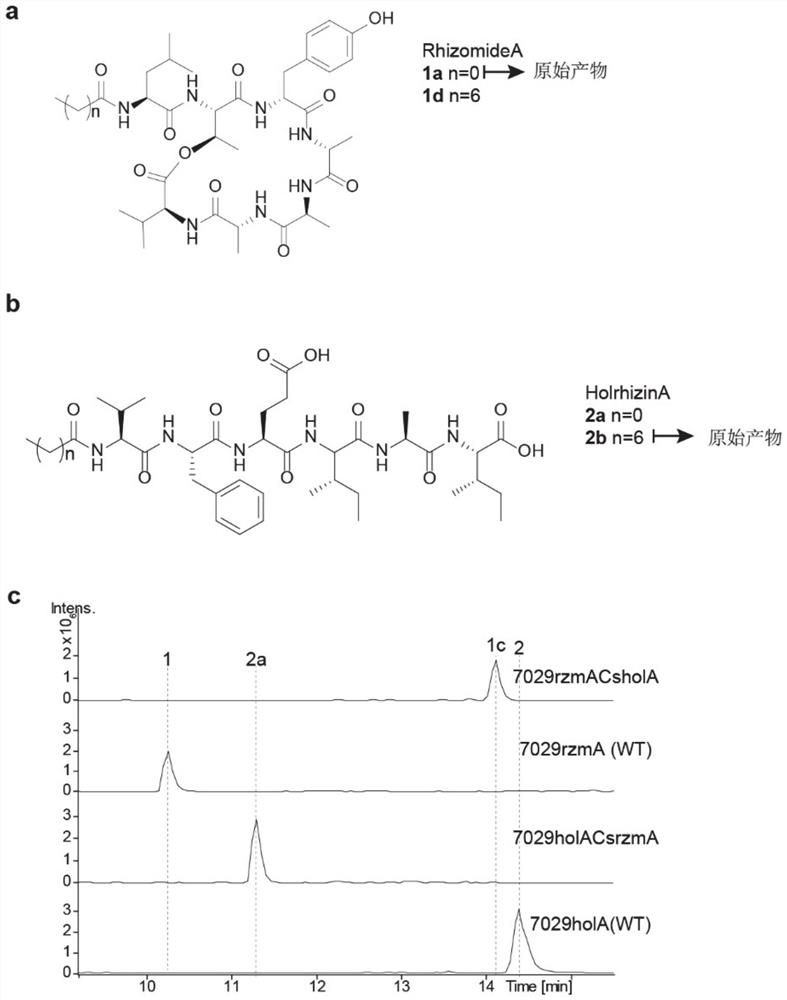
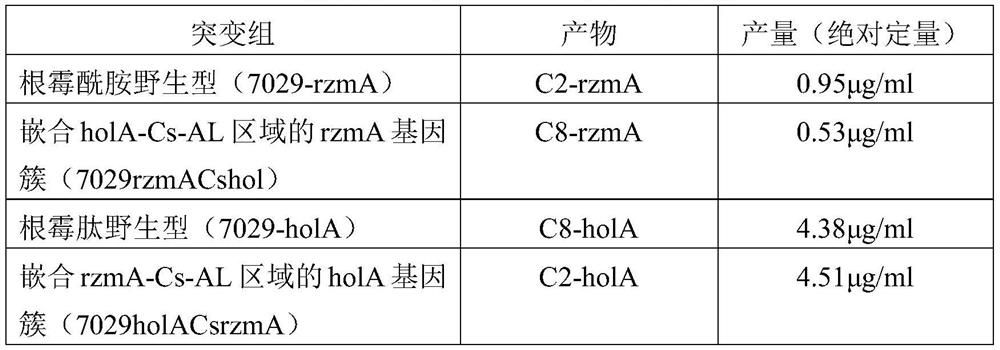
[0026] The Cs-AL regions of rzmA and holA were exchanged to obtain mutants rzmACsholA and holACsrzmA, and the catalytic activity of the mutants was verified from in vivo and in vitro levels. That is, the ability of the full-length rzmA / holA gene cluster and the corresponding mutants to catalyze the formation of the final product rzmA / holA and its derivatives shall prevail (the structure is as follows figure 1 , shown in a and b), the quantification adopts absolute quantification, after separating and purifying the derivative and wild-type lipopeptide product, it is used as a standard to quantify the mutant, and the quantitative instrument is liquid chromatography-mass spectrometry (LC-MS).
[0027] The specific implementation steps are as follows:
[0028] (1) According to antismash ( https: / / antismash.secondarymetabolites.org / #! / start ) to predict the Cs-AL region of rzmA and holA, and determine its amino acid sequence as shown in SEQ ID NO: 1 and SEQ ID NO: 2.
[0029] ...
Embodiment 2
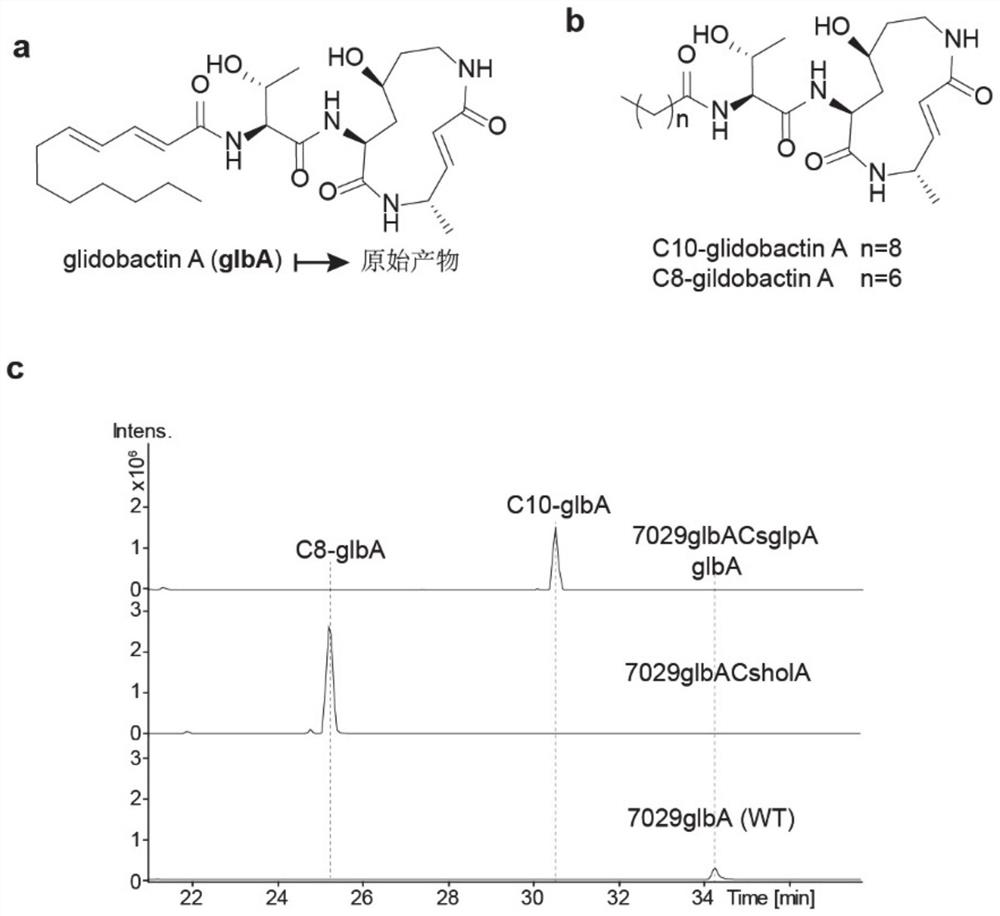
[0036] Further, the glbA gene cluster derived from DSM7029 was manipulated, and the Cs-AL region of glbA was replaced by the Cs-AL of holA from the heterologous strain DS19002, and the Cs-AL of glpA from the homologous strain DSM7029. The obtained mutants were glbACsholA and glbACsglpA, and the catalytic activity of the mutants was verified from in vivo and in vitro levels. That is, the ability of the full-length glbA gene cluster and corresponding mutants to catalyze the formation of the final product glbA and its derivatives shall prevail (the structure is as follows figure 2 , shown in a and b), the quantification adopts absolute quantification, after separating and purifying derivatives and wild-type lipopeptide products, it is used as a standard to quantify the mutants, and the quantitative instrument is liquid chromatography-mass spectrometry (LC-MS).
[0037] The Red / ET recombination system and operation in DSM7029 involved in Example 2 were all used in literature 3 (W...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com