Belantamab mafodotin in combination with pembrolizumab for treating cancer
A technology for pembrolizumab and cancer, applied in drug combinations, antibody medical components, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve problems such as breakthrough and tumor regression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0531] Example 1. Production of BCMA antigen binding proteins.
[0532] Production of antigen binding proteins according to the invention and conjugation to toxins as described herein and the respective binding affinities of such antigen binding proteins can be found in WO2012163805, as incorporated herein by reference.
Embodiment 2
[0533] Example 2 - Immunogenic Cell Death (ICD)
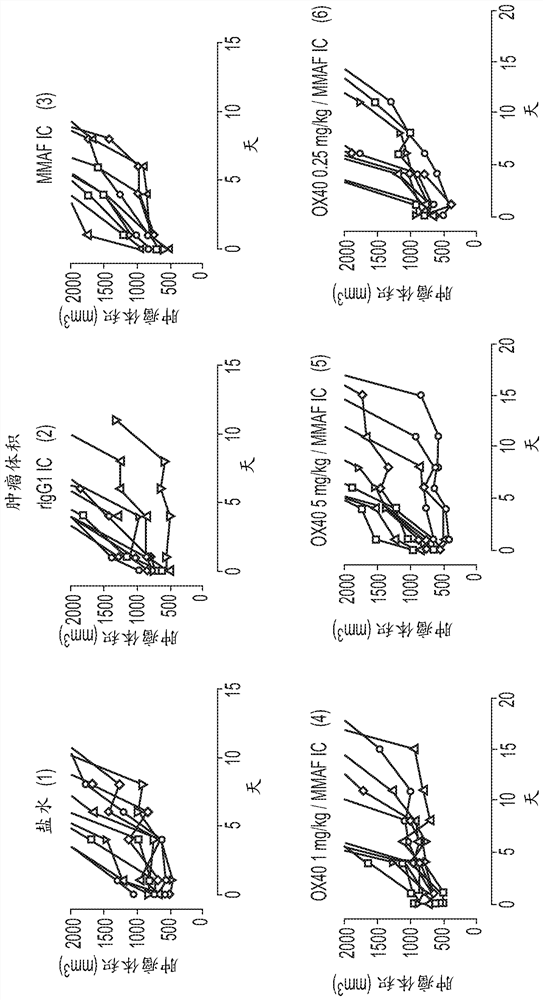
[0534] The process of immunogenic cell death can induce the production of dangerous molecules that lead to dendritic cell activation (see Figure 1A - ICD is a specific type of apoptosis that is often associated with cellular release of ATP and HMGB1 and exposure of CRT at the cell membrane. ICDs induce immune responses by participating in the process of antigen presentation by dendritic cells (DCs). NCI-H929 cells treated with BCMA ADC (anti-BCMA antibody conjugated with MMAF: GSK2857916) produced three dangerous molecules (ATP, HMGB1 and CRT) after cell killing ( Figure 1B - Tested cell lines were treated with anti-BCMA-MMAF antibody drug conjugate or mitoxantrone for 48 hours and then evaluated for: 1) loss of cell number by automated flow cytometry, 2) polyclonal Anti-CRT antibody labeling and assessment of calreticulin (CRT) exposure by flow cytometry, 3) assessment of HMGB1 content in cell supernatants by ELISA followe...
Embodiment 3
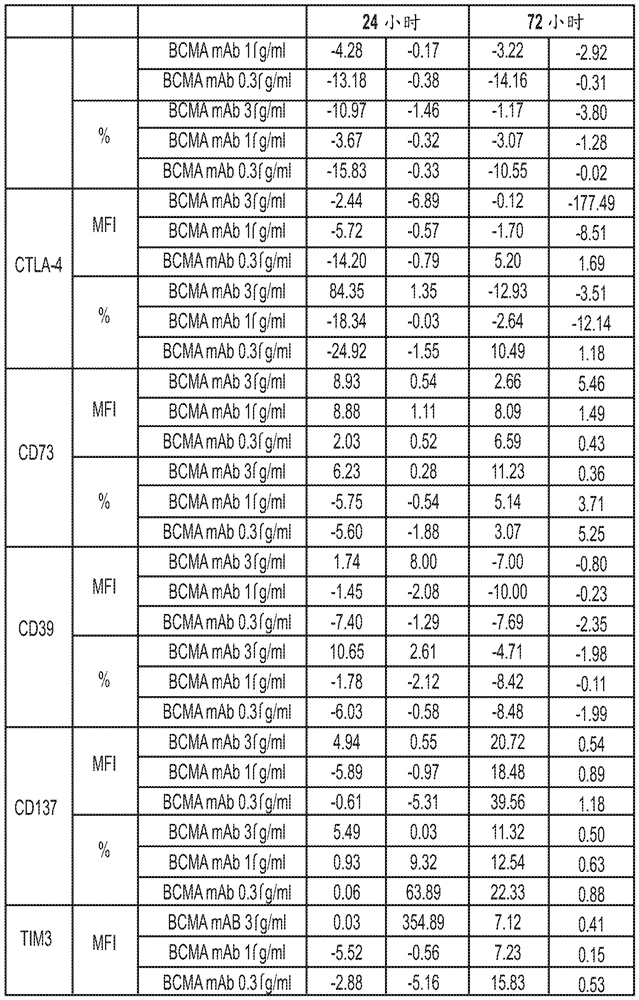
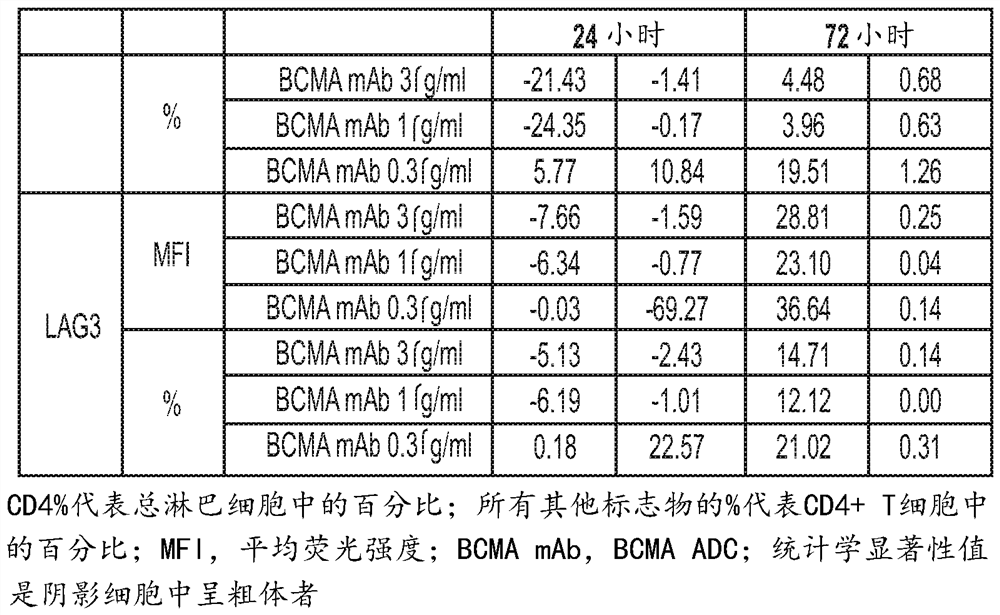
[0544] T cells can be activated by engagement of T cell receptors (TCRs) expressed on the cell surface and co-stimulatory molecules. A number of additional surface markers are upregulated following T cell activation. The in vitro effects of BCMA ADC (anti-BCMA antibody conjugated to MMAF: GSK2857916) on T cell activation and function were characterized by monitoring many of these T cell activation related markers. Furthermore, upon activation, T cells produce cytokines such as IFNγ and IL-4. The effect of BCMA ADCs on IFNγ and IL-4 production in stimulated T cells was also investigated. These experiments can provide data on the effects of BCMA ADCs on T cell activation and function. In addition, the proliferation of human CD4+ and CD8+ T cells after stimulation in the presence of BCMA ADC was also assessed. Human blood was obtained from GlaxoSmithKline's blood donor procurement program. Peripheral blood mononuclear cells (PBMC) were isolated from human whole blood by Ficol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com