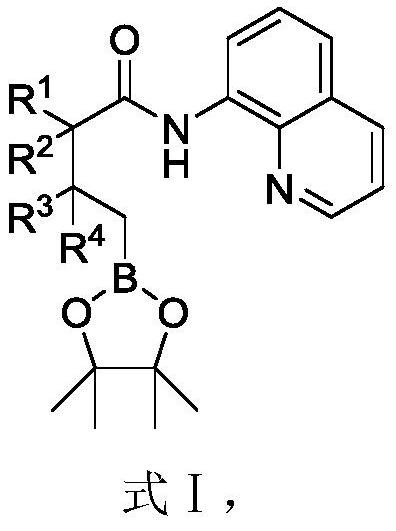
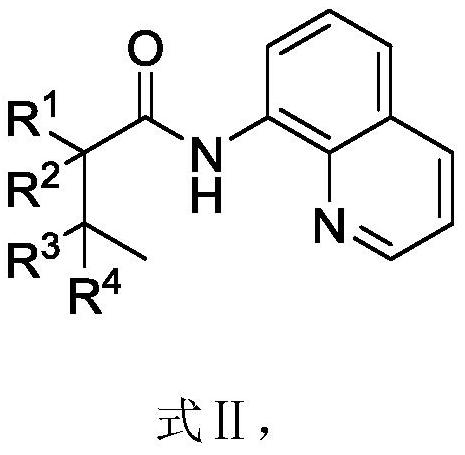
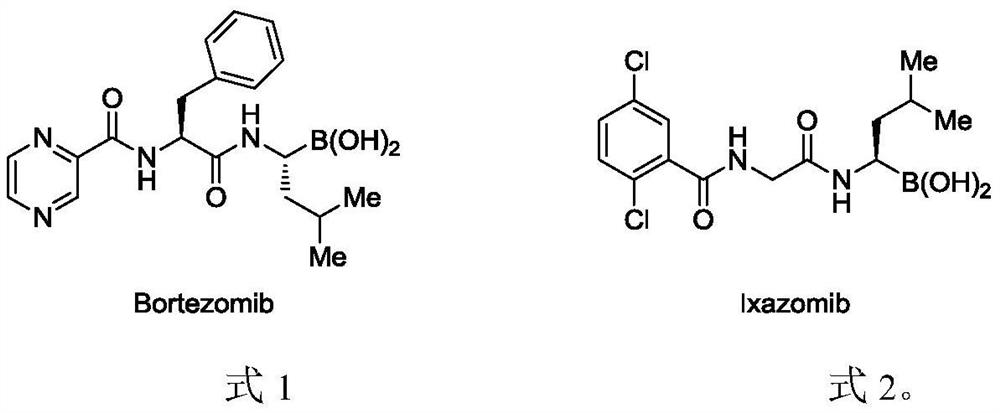
Alkylborane derivative and synthesis method thereof
A technology for alkyl borane and derivatives, applied in the field of alkyl borane derivatives and synthesis thereof, can solve the problems of complicated procedures, many reaction steps, low reaction efficiency and the like, and achieves high reaction efficiency, simple operation and easy raw materials. the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The synthetic method of alkyl borane derivatives, the steps are as follows:
[0076] In a 25 mL schlenk tube, add aliphatic carboxylic acid derivative compound 2a (0.2 mmol), bis-linked pinacolyl diborane (0.6 mmol), Pd(OPiv) in sequence under air 2 (0.02mmol), silver carbonate (0.6mmol), sodium bicarbonate (0.4mmol), 2-chloroquinoline (0.04mmol) and 2.0mL acetonitrile, stirred at 130°C for 24 hours. Separation by silica gel column chromatography (eluent is petroleum ether (60-90°C) / ethyl acetate, v / v=20:1) to obtain polysubstituted alkyl borane derivatives, denoted as 1a (32.0mg, Yield 66%). The target product was confirmed by nuclear magnetic resonance and high-resolution mass spectrometry; the synthetic route is as follows:
[0077]
[0078] Compound Characterization Data
[0079] Alkylborane (1a), yellow liquid.
[0080] 1 H NMR (400MHz, CDCl 3 ) 1 H NMR (400MHz, CDCl 3 )δ9.87(s,1H),8.80(dd,J=14.9and5.2Hz,2H),8.13(d,J=8.1Hz,1H),7.60–7.37(m,3H),2.58(s,2H ...
Embodiment 2
[0082] The synthetic method of alkyl borane derivatives, the steps are as follows:
[0083] In a 25mL schlenk tube, add aliphatic carboxylic acid derivative compound 2b (0.2mmol), double pinacol diborane (0.6mmol), Pd(OPiv) in sequence under air 2 (0.02mmol), silver carbonate (0.6mmol), sodium bicarbonate (0.4mmol), 2-chloroquinoline (0.04mmol) and 2.0mL acetonitrile, stirred at 130°C for 24 hours. Separation by silica gel column chromatography (eluent is petroleum ether (60-90°C) / ethyl acetate, v / v=20:1) to obtain polysubstituted alkylborane derivatives, denoted as 1b (48.0mg, Yield 62%). The target product was confirmed by nuclear magnetic resonance and high-resolution mass spectrometry; the synthetic route is as follows:
[0084]
[0085] Compound Characterization Data
[0086] Alkylborane (1b), yellow solid.
[0087] 1 H NMR (400MHz, CDCl 3 )δ 1 H NMR (400MHz, CDCl 3 )δ9.18(s,1H),8.98(m,1H),8.77(m,1H),8.18(m,1H),7.89(m,4H),7.68(s,1H),7.55(m,1H ),7.43(m,1H),4.48...
Embodiment 3
[0089] The synthetic method of alkyl borane derivatives, the steps are as follows:
[0090] In a 25 mL schlenk tube, add aliphatic carboxylic acid derivative compound 2c (0.2 mmol), double pinacol diborane (0.6 mmol), Pd(OPiv) in sequence under air 2 (0.02mmol), silver carbonate (0.6mmol), sodium bicarbonate (0.4mmol), 2-chloroquinoline (0.04mmol) and 2.0mL acetonitrile, stirred at 130°C for 24 hours. Separation by silica gel column chromatography (eluent is petroleum ether (60-90°C) / ethyl acetate, v / v=20:1) to obtain polysubstituted alkyl borane derivatives, denoted as 1c (62.0mg, Yield 75%). The target product was confirmed by nuclear magnetic resonance and high-resolution mass spectrometry; the synthetic route is as follows:
[0091]
[0092] Compound Characterization Data
[0093] Alkylborane (1c), yellow solid. 1 H NMR (400MHz, CDCl 3 )δ 1 H NMR (400MHz, CDCl 3 )δ8.77–8.69(m,2H),8.45(m,1H),8.19(m,1H),7.62–7.53(m,2H),7.46(t,J=7.8Hz,1H),2.19(t ,J=6.0Hz,1H),1.87(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com