Halogenated benzene ring-containing flexible large-steric-hindrance N-heterocyclic carbene palladium complex as well as preparation method and application thereof
A halogenated benzene ring, large steric hindrance technology, applied in the field of flexible large steric hindrance N-heterocyclic carbene palladium complexes and preparations, can solve the problems of limited catalyst application, low reactivity, air and water vapor sensitivity, etc., and achieve good Openness, high selective flip, excellent selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
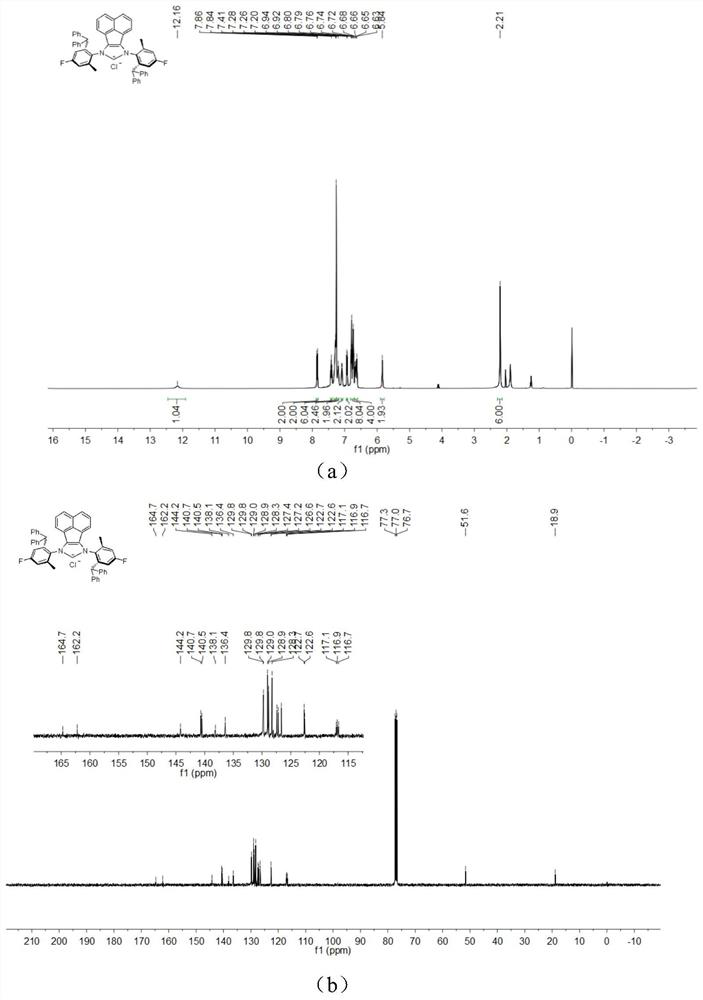
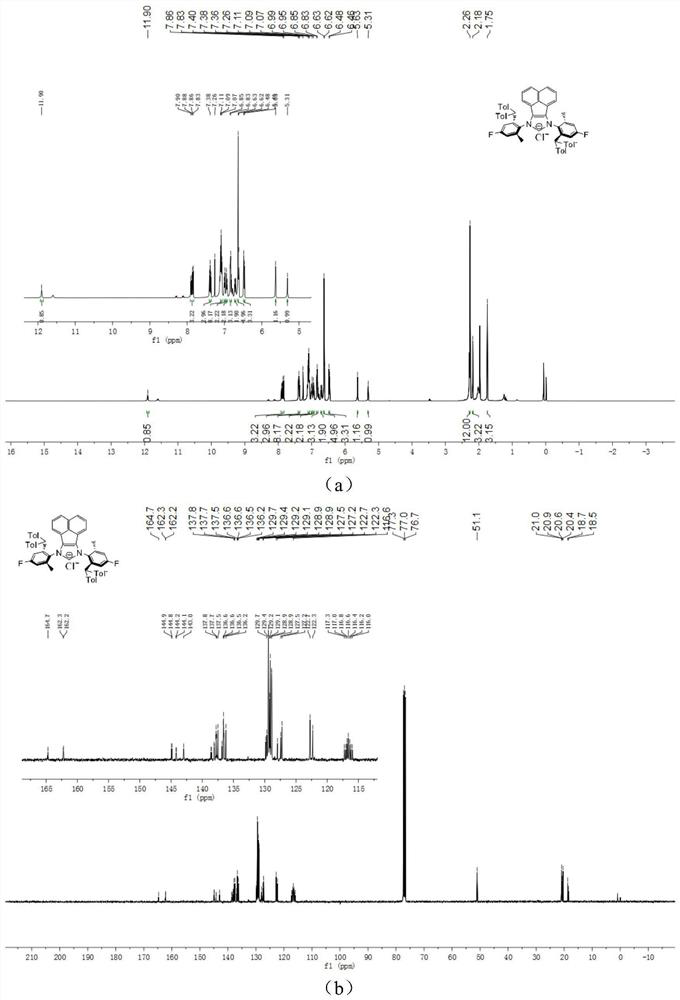
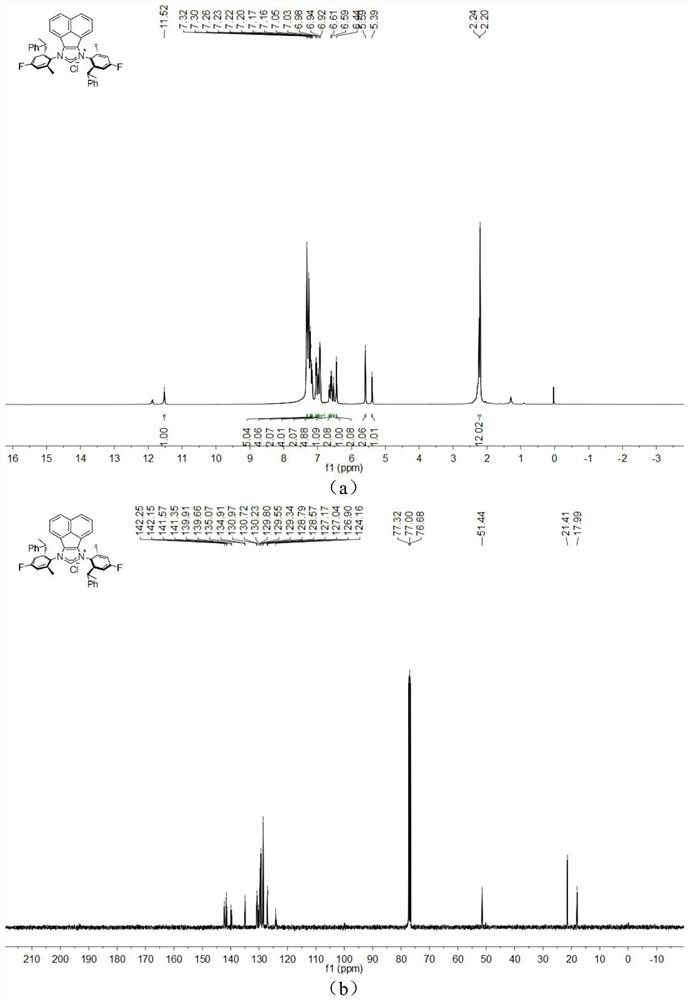
[0047] The chemical synthesis route of flexible large sterically hindered N-heterocyclic carbene palladium complexes containing halogenated benzene rings is shown below.
[0048] (1) Synthesis of α-diimine compound 4-6 (compound 4-6)
[0049]
[0050] Under the condition of nitrogen protection, acenaphthylquinone (10.0mmol), halogenated arylamine 1-3 (20.0mmol), anhydrous zinc chloride (20.0mmol) were successively added to a 100mL flask, and then 40mL of glacial acetic acid was added, and the reactant Heated to reflux for 5h, then cooled to room temperature. An orange solid was obtained by filtration, washed with a small amount of glacial acetic acid, and dried in vacuo. The obtained orange solid was dissolved in 200 mL of dichloromethane, an aqueous potassium oxalate solution was added, and stirred for 12 h. The white solid precipitate was removed, and the organic layer was dried by adding anhydrous sodium sulfate after liquid separation. The solvent was removed under r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com