Synthesis method of nano BaKL zeolite applied to aromatization of low-carbon alkane
A technology of low carbon alkane and synthesis method, which is applied in chemical instruments and methods, L-type crystalline aluminosilicate zeolite, crystalline aluminosilicate zeolite, etc. It can improve the yield of aromatics, increase the single-pass life, and improve the utilization rate of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh 12.25 g KOH in a 500 mL beaker, add 10 g pure water, stir well and add 7.06 g Al(OH) 3 , heated to 95ºC and stirred to make the solution clear. After the solution is cooled, add 117.12 g of pure water, add 0.097 g of barium nitrate, and continue stirring. Take another 500 mL beaker and weigh 49.48 g of silica sol, then use a separatory funnel to separate the clarified Al(OH) 3 The solution was added dropwise to the silica sol, and stirring was continued for 8 h. Stirring was stopped, and the gel was placed in a 200 mL crystallization kettle. The crystallization kettle was put into an oven for dynamic crystallization at 170°C for 18 h. After the crystallization is completed, the crystallization tank is taken out, and then the sample is centrifuged and washed with pure water until the pH is 7 or 8. The sample was transferred to a crucible and dried in an oven at 120 ºC for 12 h, and the yield was 91%.
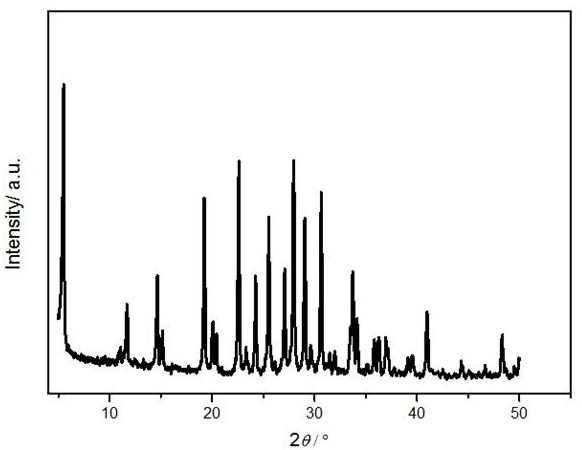
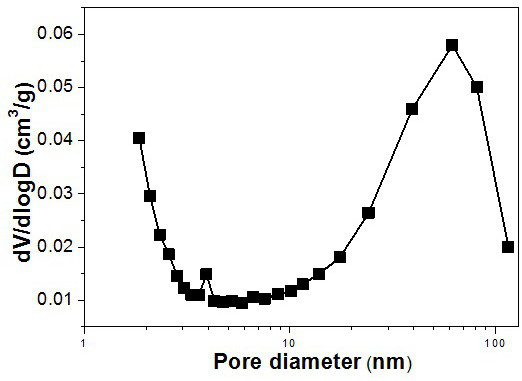
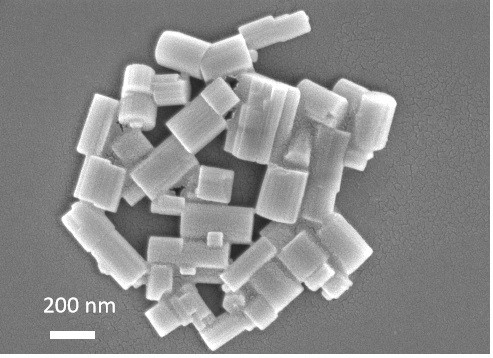
[0037] The molecular sieve is ground into powder, and anal...
Embodiment 2
[0041] Weigh 1.53 g KOH in a 100 mL beaker, add 2 g pure water, stir evenly, add 3.53 g Al(OH) 3 , heated to 95ºC and stirred to make the solution clear. After the solution is cooled, add 9 g of pure water, add 0.0061 g of barium chloride, and continue stirring. Take another 100 mL beaker and weigh 30 g of silica sol, then use a separatory funnel to separate the clarified Al(OH) 3 The solution was added dropwise to the silica sol, and stirring was continued for 24 h. Stirring was stopped, and the gel was placed in a 30 mL crystallization kettle. Put the crystallization kettle into the oven and conduct static crystallization at 185ºC for 6 h. After the crystallization is completed, the crystallization tank is taken out, and then the sample is centrifuged and washed with pure water until the pH is 7 or 8. The sample was transferred to a crucible and dried in an oven at 120 ºC for 12 h, and the yield was 67%. According to XRD spectrum analysis, the molecular sieve is BaKL. ...
Embodiment 3
[0043] Weigh 130.13 g KOH in a 2000 mL beaker, add 100 g pure water, stir well and add 30 g Al(OH) 3 , heated to 95ºC and stirred to make the solution clear. After the solution is cooled, add 1030 g of pure water, add 51.52 g of barium acetate, and continue stirring. Take another 2000 mL beaker and weigh 473.79 g of silica sol, then use a separatory funnel to separate the clarified Al(OH) 3 The solution was added dropwise to the silica sol, and stirring was continued for 4 h. Stirring was stopped, and the gel was placed in a 2 L crystallization kettle. Put the crystallization kettle into an oven, and dynamically crystallize at 110°C for 24 h. After the crystallization is completed, the crystallization tank is taken out, and then the sample is centrifuged and washed with pure water until the pH is 7 or 8. The sample was transferred to a crucible and dried in an oven at 120 ºC for 12 h, and the yield was 59%. According to XRD spectrum analysis, the molecular sieve is BaKL. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com