A kind of leech tyrosine sulfonate transferase gene and its application
A tyrosine sulfonic acid and transferase technology, applied in the field of biomedicine, can solve the problems of hindering the biosynthesis of hirudin in the analysis of natural hirudin, and the analysis work is stagnant, and achieves improved anticoagulant activity and strong substrate preference. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Mining leech tyrosine sulfonate transferase gene based on the leech transcriptome database
[0031] 1. Mining of candidate leech tyrosine sulfonate transferase genes
[0032] Through the Blastx analysis of Japanese leech genome data, the candidate gene fragment of leech tyrosine sulfonyltransferase EN-133k-group2100.Contig1 was mined. ORF program (http: / / www.ncbi.nlm.nih.gov / orffinder) was used to analyze the open reading frame of the candidate gene, and Protein Blast was used to perform homologous comparison of the database. After comparison, the sequence SEQ ID NO. 3 was determined to be The nucleotide sequence of alternative leech tyrosine sulfonate transferase, SEQ ID NO. 1 is the amino acid sequence of alternative leech tyrosine sulfonate transferase.
[0033] 2. Construction of leech cDNA library
[0034] After the Japanese medical leech was fed with fresh duck blood for 2 h, the above leech was quick-frozen in liquid nitrogen. After grinding with liq...
Embodiment 2
[0037] Example 2 Preparation of leech tyrosine sulfonate transferase by using microbial cells
[0038] 1. Preparation of leech tyrosine sulfonate transferase by recombinant Escherichia coli
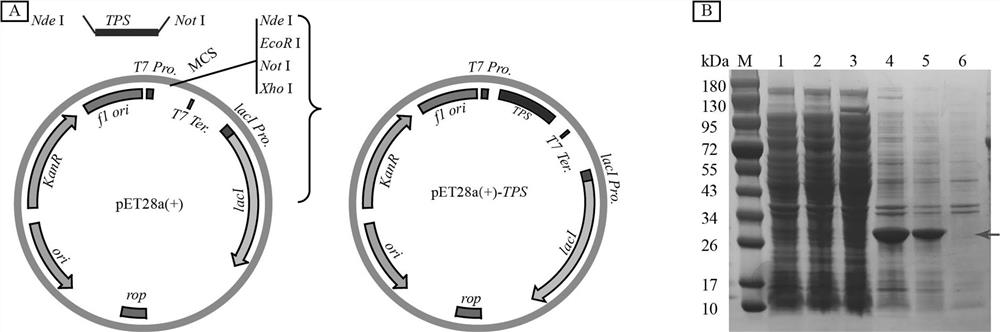
[0039] In view of the codon preference of microbial cells when expressing proteins, in order to obtain high-efficiency expression of the leech tyrosine sulfonate transferase gene, we artificially synthesized the codon-optimized leech tyrosine sulfonate transferase gene TPS , assembled by Gibbson, integrated into pET28a (+) by homologous recombination Nde I and not I site, construct the recombinant expression vector pET28a(+)- TPS ,in TPS The sequence is shown in SEQ. ID NO. 2. Transformation using the above-mentioned recombinant expression vector E . coli BL21 Obtaining Escherichia coli Recombinant Expression Strain of Leech Tyrosine Sulfonyltransferase E . coli BL21 pET28a(+)- TPS , for the subsequent preparation of leech tyrosine sulfonyltransferase ( image 3 in A...
Embodiment 3
[0044] Example 3 Establishment of leech tyrosine sulfonate transferase catalytic system
[0045] 1. Purification of leech tyrosine sulfonyltransferase
[0046] After equilibrating the Ni-NTAAgarose Fast Flow column with Buffer A (20 mM Tris-HCl, 0.5 M NaCl, 20 mM imidazole, pH 7.4), permeate the leech tyrosine sulfotransferase crude enzyme solution 1 and 2 respectively through 0.22 μm After filtration, the supernatant was passed through the Ni-NTA Agarose Fast Flow column at a rate of 3 mL / min. After binding for 10 min, Buffer B (20 mM Tris-HCl, 0.5 M NaCl, 500 mM imidazole, pH 7.4) was used to elute Enzyme active fractions were collected, concentrated, and freeze-dried.
[0047] 2. Preparation of natural hirudin
[0048] Using recombinant hirudin 1 (Sigma) or recombinant hirudin 2 (Sigma) as raw materials, the reaction solution includes 1-200 mMTris-HCl (pH5.0), 0.1-50 mM mM PAPS, 0.1-100 μM leech tyrosine sulfonate Acid transferase, 0.1-50 mM recombinant hirudin, 10-50°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com