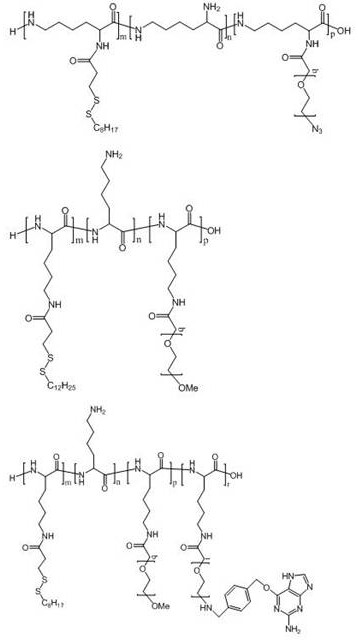
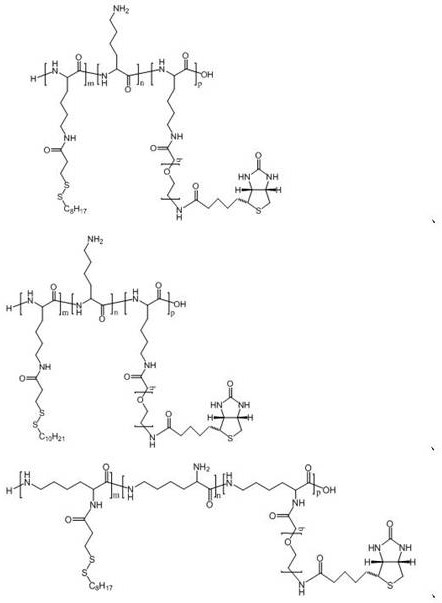
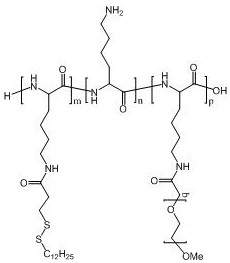
Polyamino acid for targeted delivery of mRNA vaccine and preparation method and application of polyamino acid
A polyamino acid and amino acid technology, applied in the field of polymer materials, can solve the problems of inability to achieve effective delivery of mRNA vaccines and drugs, and low delivery efficiency, and achieve good biocompatibility and targeted delivery efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0131] The preparation method of the present invention will be further described in detail in conjunction with specific examples below. It should be understood that the following examples are only for illustrating and explaining the present invention, and should not be construed as limiting the protection scope of the present invention. All technologies realized based on the above contents of the present invention are covered within the scope of protection intended by the present invention.
[0132] The experimental methods used in the following examples are conventional methods unless otherwise specified; the reagents and materials used in the following examples can be obtained from commercial sources unless otherwise specified.
[0133] The buffer solution used in the following examples is a commonly used buffer solution, including phosphate buffer solution, HEPES buffer solution and Tris buffer solution, and the water is MiliQ deionized water after autoclave sterilization, ...
Embodiment 1
[0136] Example 1 Preparation of polyamino acid PLL-SSMO25-PEG2000-Biotin20
[0137] Weigh 10 mg of α-poly-L-lysine hydrobromide (hydrobromide of PLL, average molecular weight 22,500 Daltons), and dissolve it in 150 µL of phosphate buffer (pH 7.4) to obtain a solution ( A). Weigh 20 mg of polyethylene glycol 2000 (BIOTIN-PEG2000-SCM, polyethylene glycol with an average molecular weight of 2000 Daltons) modified with biotin and succinimide carboxymethyl ester, purchased from Beijing Jiankai Technology Co., Ltd. company), which was dissolved in 150 µL tetrahydrofuran (analytical grade, Nanjing Reagent Company). To the resulting solution was added 3.8 mg of succinimide 3-(2-pyridyldithio)propionate (SPDP, molecular weight 312, Thermo Fisher) coupling agent to obtain a mixture solution (B). Mix solution (A) and solution (B) in a test tube, shake and react at room temperature for 4 hours, then add 1.2 times the reaction equivalent of n-octyl mercaptan 2.5 µL (MO, analytical grade,...
Embodiment 2
[0140] Example 2 Preparation of polyamino acid PLL-SSMO25-PEG3500-Biotin20
[0141]Weigh 10 mg of PLL hydrobromide (average molecular weight 22,500 Daltons) in a 2 mL centrifuge tube and dissolve it in 150 µL of phosphate buffer (pH 7.4) to obtain solution (A). Weigh 33 mg of polyethylene glycol 3500 (BIOTIN-PEG3500-SCM, polyethylene glycol with an average molecular weight of 3500 Daltons) modified with biotin and succinimide carboxymethyl ester, purchased from Beijing Jiankai Technology Co., Ltd. company), dissolved in 150 µL THF. To the resulting solution was added 3.8 mg of succinimide 3-(2-pyridyldithio)propionate (SPDP, molecular weight 312, Thermo Fisher) coupling agent to obtain a mixture solution (B). Mix solution (A) and solution (B) in a test tube, shake and react at room temperature for 4 hours, then add 1.2 times the reaction equivalent of n-octyl mercaptan 2.5 µL (MO, analytical grade, molecular weight 146, density 0.84 g / L, Beijing Bailingwei Company). The rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| critical micelle concentration (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com