Application of gene editing technology in cancer treatment
A use, breast cancer technology, applied in the direction of gene therapy, application, genetic engineering, etc., can solve the problem of limited options, and achieve the effect of good inhibition and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 specific sgRNA
[0038] According to the gene sequence of human VEGF, using the design rules of gRNA, the sgRNA sequence was designed and prepared in a manual and online way.
[0039] In order to enhance the specificity of gene knockout and specifically knock out the coding region that destroys VEGF, this experiment adopted a 2hitKO strategy for gene knockout, that is, designing one sgRNA at an appropriate position upstream and downstream of the coding region, and then assembling the two sgRNAs into the same Cas9 Expression vector. Two sgRNA sequences were designed for the VEGF gene, and a BsmBI restriction site was added at the 5' end of the sense and antisense strands of the sequences. The designed sgRNA primers are shown in Table 1.
[0040] Table 1 The sequence of sgRNA primers for specific knockout of VEGF gene
[0041] Primer name Primer sequence (5'-3') bp VEGF-sgRNA1-F CACCGTCTACCTCCACCATGCCAAG 25 VEGF...
Embodiment 2
[0043] Construction and transfection of embodiment 2 expression vector
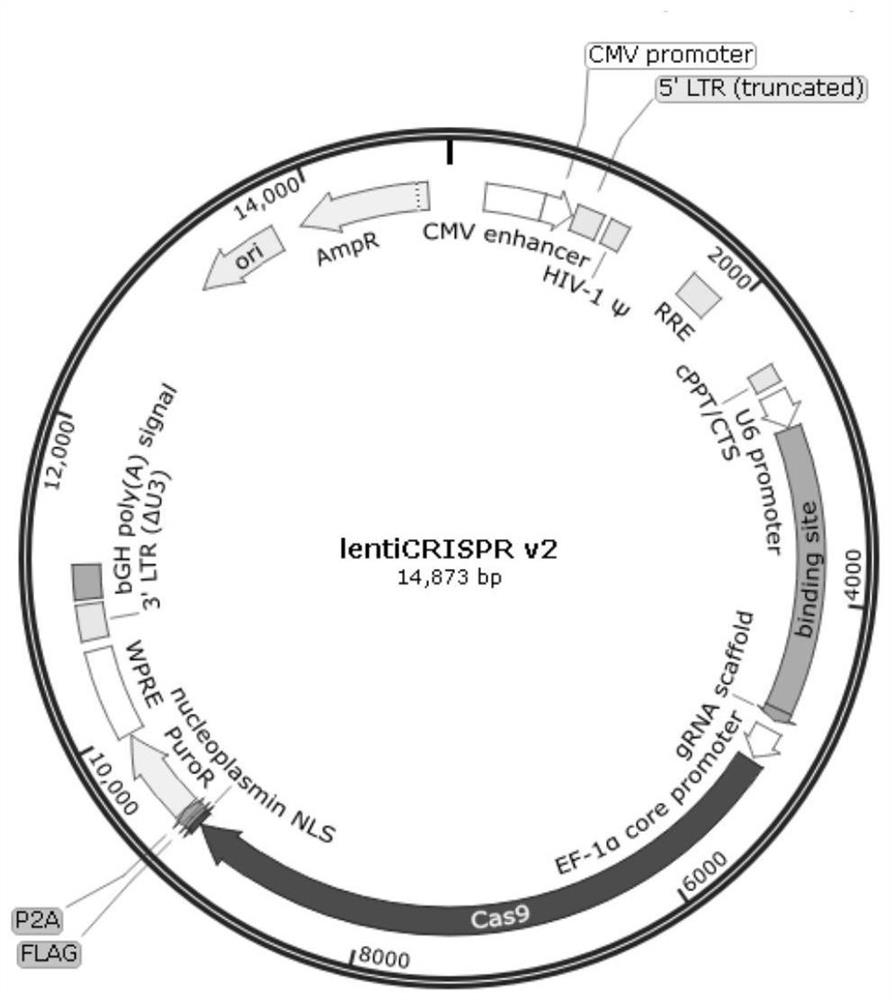
[0044] The two sgRNAs prepared by annealing in Example 1 were respectively digested with BsmBI, and then mixed and ligated with the purified lentiCRISPR v2 vector (the LentiCRISPR V2 vector contains a U6 promoter that guides gene transcription, Cas9 nuclease, ampicillin, etc. components, see figure 1 shown). Under the action of T4 DNA ligase, ligate at 16°C for 16h. Take the ligation product and transform it into 100 μl DH5α competent bacteria, screen the positive clones with ampicillin resistance, pick a single clone, and send it to BGI for sequencing. The gene sequence was identified and the construction was successful.
[0045] Human ovarian cancer cell line SK-OV-3 (product number EY-X0733, Shanghai Yiyan Biotechnology Co., Ltd.), cultured in RPMI 1640 full culture medium containing 10% fetal bovine serum, at a constant temperature of 37°C and a saturated humidity of 5% CO2 Subculture in the incub...
Embodiment 3
[0047] Example 3 Cell Identification
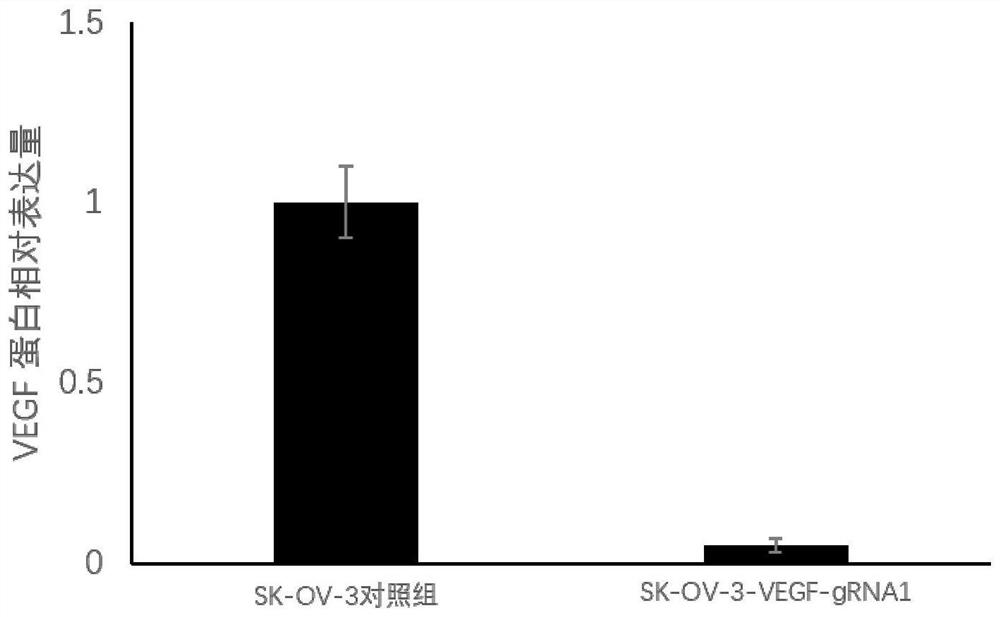
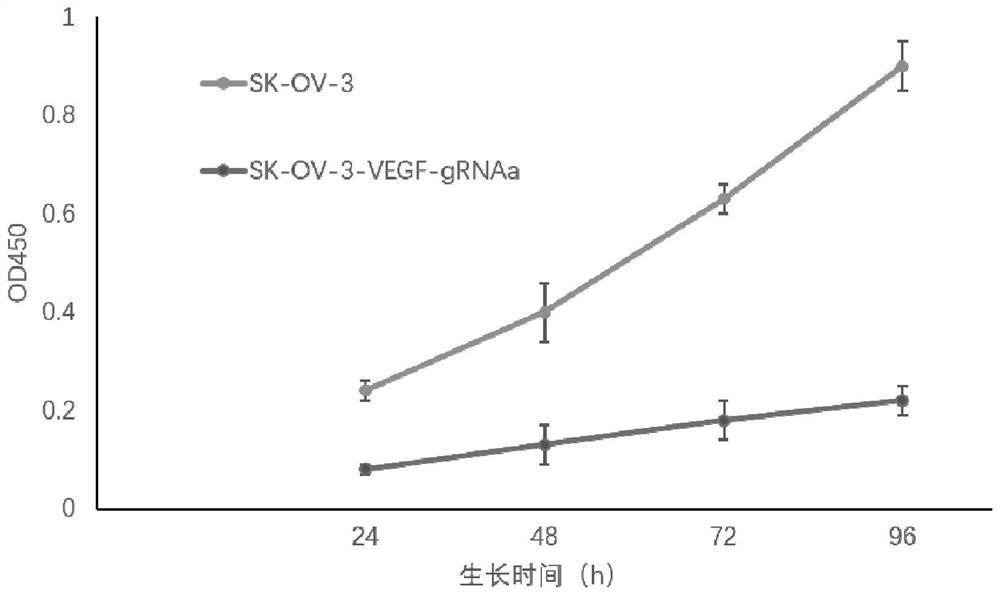
[0048] Dilute the cells screened in Example 2 into a 96-well plate, digest with trypsin, collect the overgrown SK-OV-3 cells, resuspend in PBS, add 4×SDS lysate, boil in water bath for 15 minutes, and centrifuge at 12000r / min to collect Cleared for polyacrylamide gel electrophoresis. After electrophoresis, transfer to PVDF membrane, block with 5% skimmed milk powder at room temperature for 1 h, then add endogenous VEGF antibody at 1:500 and incubate at room temperature for 1 h; wash membrane with 1×TBST for 3 times, 5 min each time, use HRP-labeled goat antibody Mouse IgG (1:5000) was incubated at room temperature for 1 h, the membrane was washed 3 times with 1×TBST, 5 min each time, and the Western blot bands were detected by chemiluminescence. Normal cultured SK-OV-3 cells were used as control. The result is as figure 2 shown.
[0049] from figure 2 The results showed that the VEGF protein in the gene-edited cells was not express...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com